Figure 2. Different classes of covalent inhibitors.
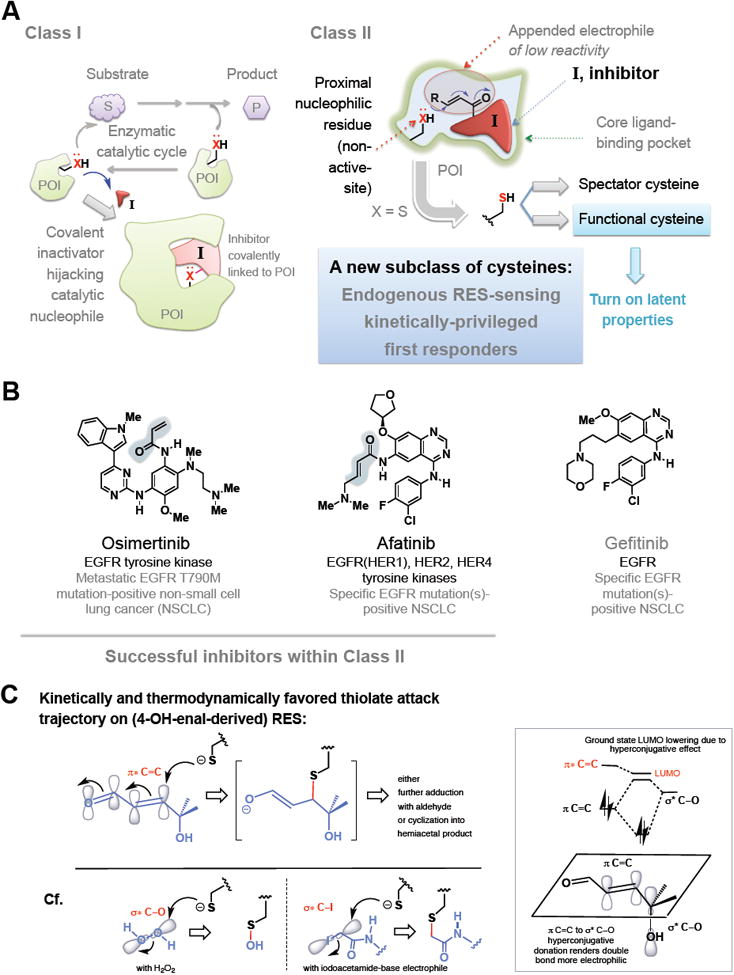
(A) Class I: active-site blockers. These electrophiles bind at the active site and engage chemically with active-site nucleophile to form an inhibited complex. Class II: These bind reversibly but are trapped out by a proximate nucleophilic residue (principally cysteine) not part of the catalytic chemistry step. This cysteine could either be a rank-and-file residue (see text), or a functional kinetically-privileged first-responding RES/ROS-sensor. Also see Figure 3.
(B) Representative inhibitors in Class II and their protein targets and disease. Blue shade marks the appended electrophile. For representative inhibitors in Class I, see carfilzomib/bortezomib in Figure 1B. Gefitinib is often considered as a non-covalent analog of afatinib.
(C) Different stereoelectronic requirements for chemical bond formation events between sp2-carbon-derived RES-based electrophiles such as HNE and sp3-based oxidants and electrophiles such as H2O2 and iodoacetamide(IA)-derivatives, respectively. With HNE, the allylic hydroxyl group enhances the partial positive charge (thus electrophilicity) at the Michael acceptor (sp2-hybridized) carbon through simultaneous hyperconjugation and inductive electron-withdrawing effects from electronegative oxygen. Inset shows hyperconjugation in ground state including molecular orbital mixing effect, resulting in overall lowering of the lowest unoccupied molecular orbital (LUMO) (π* of enal). The transition state (TS) of the reaction favors Felkin-Ahn type addition wherein σ*C—OH orients anti-periplanar to the forming thiolate carbon bond as shown. Negative charge in the TS is delocalized over what is formally an allylic anion-type intermediate: this factor contributes significantly to TS-stabilization in RES–thiolate addition, and recognition likely plays a role in kinetically-privileged RES-sensing. [The square bracket shows the first-formed intermediate, which progresses to yield indicated product(s) (see, for example, Long and Aye 2016) ]. By contrast, thiolate addition to H2O2 and IA-derived ROS and RES, respectively, has less negative-charge delocalization in the TS.
