Fig. 3.
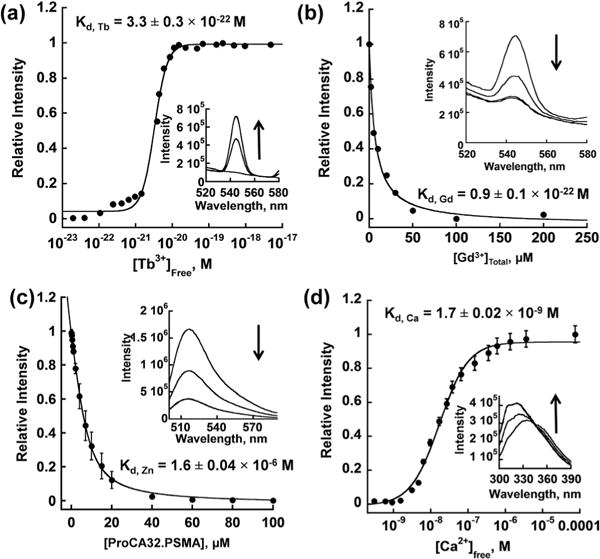
Determination of Tb3+, Gd3+, Zn2+ and Ca2+ affinity to ProCA32. PSMA. (a) Determined Tb3+ affinity to ProCA32.PSMA using the Tb-DTPA chelator buffer system. Free [Tb3+] was maintained in a range between 10−23 and 10−17 M by a tightly controlled concentration ratio between Tb-DTPA and free DTPA. The interaction between Tb3+ and ProCA32. PSMA was quantified by fluorescence intensity increase mediated by the luminescence resonance energy transfer between Trp in ProCA32.PSMA and bounded Tb3+. (b) Determined Gd3+ affinity by using Gd3+ competition assay. Different concentrations of Gd3+ were incubated with Tb3+-loaded ProCA32.PSMA. The fluorescence intensity caused by the luminescence resonance energy transfer between Trp in ProCA32.PSMA and bounded Tb3+ decrease when Gd3+ competed Tb3+ out of the metal binding pocket. (c) Determined Zn2+ affinity using Fluozin-1 competition assay. (d) Determined Ca2+ affinity to ProCA32.PSMA using the Ca2+-EGTA buffer system. Free [Ca2+] was maintained in a range between 10−10 and 10−4 M by the tightly controlled concentration of Ca2+ and EGTA. The interaction between Ca2+ and ProCA32.PSMA was monitored by the Trp fluorescence intensity increase by increasing Ca2+ concentration.
