Figure 7.
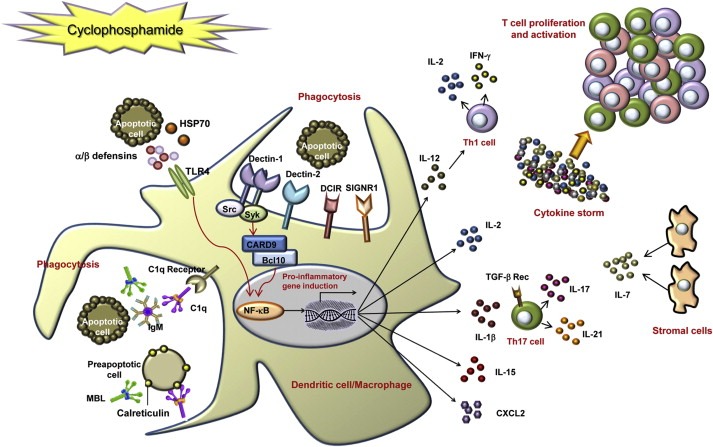
Model of cyclophosphamide (CTX)‐mediated immunomodulation on the basis of genes up‐regulated by treatment. CTX treatment of tumor‐bearing mice results in the killing of proliferating cells, including tumor and host bone marrow and spleen cells. Pre‐apoptotic and dead cells release or expose danger signals such as HSP70, alarmins (defensin‐alpha‐4 and defensin‐beta‐2) and calreticulin. The latter could be recognized by the soluble pattern recognition receptors (PRRs) C1q and mannose binding lectin (MBL), thus triggering phagocytosis of apoptotic cells. The secretion of HSP70 and defensin‐beta‐2, through the activation of TLR4, may act directly on immature DC, inducing their maturation and up‐regulation of costimulatory molecules, via activation of NF‐κB. Apoptotic cells may be recognized also by membrane PRRs, such as dectin‐1, dectin‐2, DCIR and SIGNR1. After ligand binding, the C‐type lectin dectin‐1 activates the transcription factor NF‐κB through a Syk kinase‐dependent signaling pathway, leading to the induction of genes encoding proinflammatory cytokines and chemokines. Up‐regulated cytokines, including IL‐1β, IL‐2, IL‐7, IL‐15, IL‐17, IL‐21 and IFN‐γ (produced either by DC or by T cells or by stromal cells) may therefore induce Th1 and Th17 polarization and, altogether, T cell proliferation and activation (Moschella et al., 2011).
