Figure 1.
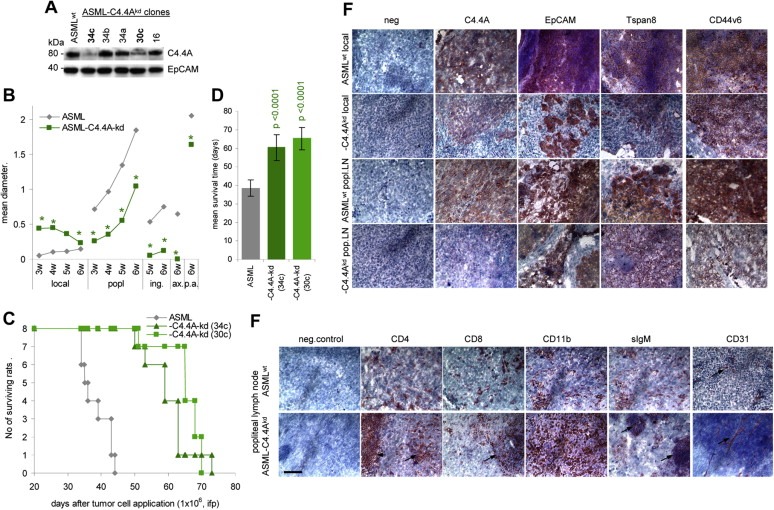
Retarded metastasis formation of ASML‐C4.4Akd cells: (A) WB of C4.4A in ASML and ASML‐C4.4Akd cells. EpCAM served as control. Clones 34c and 30c were used throughout, presented data mostly derived from clone 34c. (B‐D) BDX rats received 1 × 106 ASML or ASML‐C4.4Akd (clones 34c and 30c) cells, ifp. (B) Local tumor growth and growth in draining (popliteal) and distant (inguinal, paraaortic < p.a.>, axillary) LN during 6wk after tumor cell application. The mean diameter of 5 rats/group is shown. Significant differences between ASML and ASML‐C4.4Akd cells: *. (C) Survival time and survival rate of ASML and ASML‐C4.4Akd bearing rats (D) Mean survival timeSD of 8 rats/group; p‐values are shown. (E and F) Immunohistology of the local tumor and popliteal LN metastasis of ASML and ASML‐C4.4Akd‐bearing rats. Shock frozen footpad and popliteal LN sections were stained with the ASML markers C4.4A, EpCAM, Tspan8 and CD44v6 and (popliteal node) the leukocyte markers CD4, CD8, CD11b (M), sIgM (B cells) and the endothelial cell marker CD31. Scale bar: 100 m. In (F) staining of CD4+ and CD8+ cells in the perifollicular region and of B cell follicles in ASML‐C4.4Akd tumor bearers are indicated by an arrow. In ASML tumor bearers the lymph node structure is destroyed. Instead, ASML‐C4.4Akd tumor nodules are well vascularized, while only short stretches of endothelial cells are seen in ASML tumors (arrows). Metastasis formation of ASML‐C4.4Akd cells is delayed compared to ASML cells. ASML‐C4.4Akd cells do not invade the surrounding tissue and do not interfere with endothelial cell sprouting.
