Figure 2.
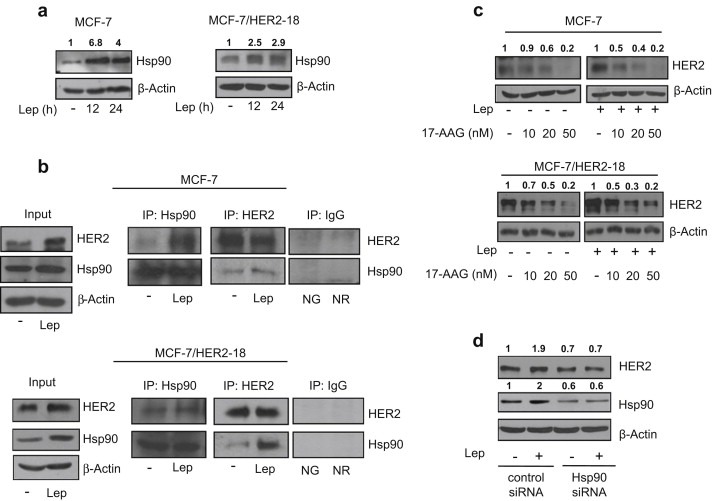
Leptin enhances Hsp90 expression. (a) Immunoblotting analysis of Hsp90 levels in total protein extracts from MCF‐7 (left panel) and MCF‐7/HER2‐18 (right panel) cells untreated (−) or treated with leptin 500 ng/ml (Lep) as indicated. (b) MCF‐7 (upper panel) and MCF‐7/HER2‐18 (lower panel) cells were untreated (−) or treated with Lep for 24 h before lysis. Hsp90 and HER2 proteins were immunoprecipitated using anti‐Hsp90 (IP:Hsp90) and anti‐HER2 (IP:HER2) antibodies respectively and resolved in SDS–polyacrylamide gel electrophoresis. Immunoblotting was performed using anti‐HER2 and anti‐Hsp90 antibodies respectively. Whole‐cell lysates (Input) were used as input controls. Negative control was performed by incubation of cell lysates with protein A/G agarose and normal goat (NG) or rabbit (NR) antisera. (c) Immunoblotting analysis of HER2 from total extracts of MCF‐7 (upper panel) and MCF‐7/HER2‐18 (lower panel) cells treated for 24 h with Lep (500 ng/ml) in the presence or not of growing doses (10–20 and 50 nM) of the selective Hsp90 inhibitor 17‐allylamino‐17‐demethoxygeldanamycin (17‐AAG). (d) Total cellular proteins were isolated from MCF‐7 cells transfected with Hsp90 siRNA or control siRNA and treated for 24 h with Lep. Equal amounts of total cellular extracts were analyzed for HER2 and Hsp90 protein levels by immunoblotting. β‐Actin was used as loading control. Numbers on top of the blots represent the average fold change versus untreated cells normalized for β‐actin.
