Figure 6.
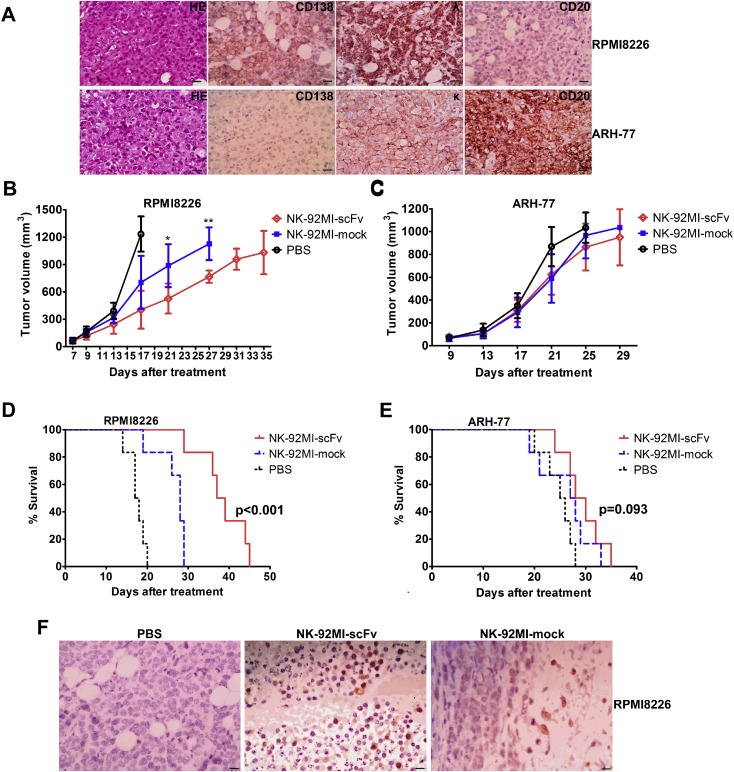
Improved anti‐MM efficacy induced by irradiated NK‐92MI‐scFv against CD138‐positive MM cell in xenograft model. Mice challenged subcutaneously with RPMI8226 or ARH‐77 cells (5 × 106). When palpable tumors (≥5 mm in diameter) developed, mice inoculated with RPMI8226 or ARH‐77 were randomly assigned into three treatment groups (6 per group) receiving intravenously injection of 4 × 107 10Gy –irradiated NK‐92MI‐scFv or NK‐92MI‐mock or the vehicle alone (PBS) via tail vein every other day for a total of three injections. (A) Representative immunohistochemistry slides of subcutaneous xenograft sections for RPMI8226‐ and ARH‐77‐challenged mice. Magnification: × 400. (Scale bar: 20 μm); Caliper measurements of the greatest length and width were performed on alternate days to estimate the tumor volume using the following formula: 4π/3 × (tumor width/2)2 × (tumor length/2). The tumor volume of mice challenged with RPMI8226 (B) or ARH‐77 (C) was expressed as mean ± SD. Mice were humanely sacrificed when subcutaneous tumors reached 15 mm in diameter. Survival was evaluated from the first day of treatment until death. Survival were shown for mice challenged with RPMI8226 (D) or ARH‐77 (E). (F) Human NK cells were detectable in appreciable numbers in the MM tumor bed of sacrificed mice 29 days after adoptive transfer. Immunohistochemical analysis of subcutaneous xenograft sections of RPMI8226‐ challenged mice 29 days after injection of NK‐92MI‐scFv or NK‐92MI‐mock. The NK cells were stained brown with rabbit anti‐human CD2 antibody using the LSAB + kit (DAKO, Carpinteria, CA, USA). Nuclei are stained with hematoxylin. Magnification: × 400. (Scale bar: 20 μm) *p < 0.05, **p < 0.01 compared with mice treated with NK‐92MI‐mock.
