Figure 2.
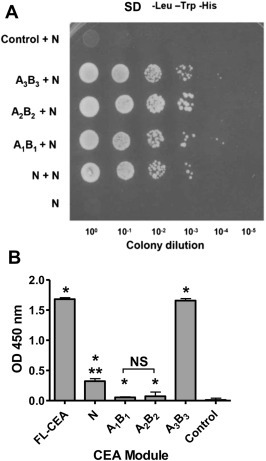
Mapping of the CEA segments responsible for homotypic interactions. A. Yeast‐2‐hybrid analysis of the interacting human CEA modules. A “bait” vector was constructed where the IgV‐like N domain of CEA was fused to the GAL4 DNA‐binding domain. The yeast strain AH109 was co‐transformed with this vector as well as with a vector expressing either the IgV‐like N‐, IgC‐like A1B1‐, A2B2‐, or A3B3‐domains fused to the GAL4 activation domain. The resulting yeast colonies were grown overnight and spotted (5 μl) as tenfold serial dilution onto SD medium lacking Trp, Leu, and His to select for interacting partners leading to colony growth. The lack of interaction between CEA N (as bait) and rhRPLP2 (as prey) served as a control. B. Confirmation of the homotypic interactions between the FLAG‐tagged N‐ and the Ig domains of CEA, or rhTNF‐α (control protein). Binding of FLAG‐rCEA N to either immobilized rCEA Ig domains, the full‐length tumor glycoform of CEA or to rhTNF‐α (negative control) was monitored by ELISA. The presence of bound FLAG‐tagged N domain was detected using an HRP‐coupled anti‐FLAG M2 (1:5000. NS: not statistically significant); * significant when compared to the control protein (P ≤ 0.05); ** significant when comparing the binding intensity of rCEA N to either A1B1 or A2B2. Statistical significance was established using a Student‐t‐test.
