Figure 1.
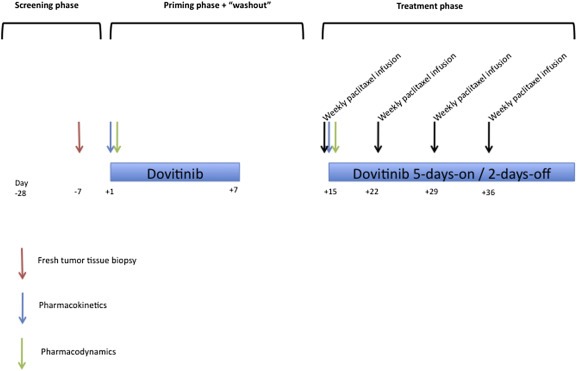
Treatment schedule. The screening included conventional assessments plus an image‐guided tumor biopsy (within 7 days before to the first dovitinib dose) and LVEF determination. Dovitinib was then administered orally for a week (“priming phase”); a pharmacokinetic profile was obtained on an inpatient basis on day 1. Blood samples for pharmacodynamic determinations were obtained at 0 and +24 h. After a 1 week “wash‐out” period, the treatment phase started with concurrent treatment of dovitinib + paclitaxel. A second pharmacokinetic profile (so that the paclitaxel‐induced modifications in dovitinib pharmacokinetics could be studied) including as well paclitaxel determinations, in order to compare the pharmacokinetics in combination with dovitinib with historical data of weekly paclitaxel (Fennelly et al., 1997), and two additional samples for pharmacodynamic determinations were obtained. The patients were discharged after 24 h and the treatment was continued with dovitinib administered orally on a schedule of 5‐days‐on/2‐days‐off in combination with weekly paclitaxel, in 28‐days cycles. Patients were visited and evaluated for toxicity weekly beginning with the first dose of dovitinib and until the end of the first cycle, bi‐weekly during the second and third cycles, and subsequently on a monthly basis. RECIST evaluations were performed every 2 cycles.
