Figure 4.
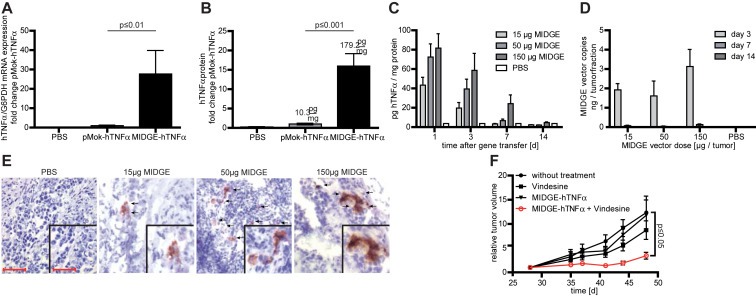
In vivo efficiency and safety analysis of nonviral gene transfer in the A375 melanoma xenograft model. (A) The intratumoral in vivo jet‐injection of the hTNFα encoding MIDGE‐hTNFα vector leads to increased transgene expression at the mRNA level compared to the parental plasmid. (B) The intratumoral in vivo jet‐injection of the hTNFα encoding MIDGE‐hTNFα vector leads to high‐level cytokine production. (C) Detailed ELISA analysis of hTNFα protein expression over time. Within 14 days hTNFαprotein expression declines even at the highest vector dose. (D) Intratumoral vector clearance was monitored over time with qPCR. Clearance is completed within 14 days. (E) Immunohistochemistry staining of A375 melanoma xenografts after treatment with PBS and three different doses of MIDGE‐hTNFα vector 24 h after gene transfer. Scale bar represents 50 μm and 25 μm (inset). (F) The in vivo combination of intratumoral jet‐injection MIDGE‐hTNFα gene transfer and vindesine leads to a significantly reduced tumor growth. The values are in mean ± SEM. One way ANOVA followed by Bonferroni's Multiple Comparison Test was used for statistical analysis. For the combination experiment the Dunnett's Multiple Comparison Test vs. untreated group was used.
