Figure 1.
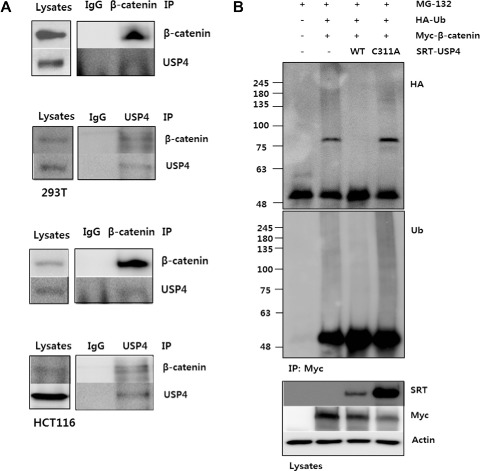
USP4 binds β‐catenin controls its stability. (A) Interaction between USP4 and β‐catenin in 293T and HCT116 cells. Four hours prior to harvest, 10 μM MG132 was added to inhibit proteasomal degradation. The cells lysates were immunoprecipitated using anti‐β‐catenin and anti‐USP4 antibodies, respectively. The cell lysates and IP eluents were immunoblotted using anti‐β‐catenin and anti‐SRT antibodies. IgG was used for control. (B) DUB activity of USP4 and its active‐site mutant C311A on Ub‐β‐catenin. 293T cells transfected with indicated DNAs were culture for 24 h. MG132 (10 μM) was added 4 h before harvest. The cell lysates was immunoprecipitated with anti‐Myc antibody under denaturing condition. The ubiquitination level of immunoprecipitants was measured by immunoblotting using anti‐HA and anti‐Ub antibodies, respectively. (C) The effect of wild type and C311A USP4 on β‐catenin stability. Wild‐type or C311A SRT‐USP4 (+: 0.2 μg, ++: 0.5 μg) was co‐transfected with Myc‐β‐catenin (0.5 μg) into 293T cells. After 48 h the levels of Myc‐β‐catenin and SRT‐USP4 were evaluated by immunoblotting. (D) The effect of USP4 siRNA (siUSP4) on ubiquitination of β‐catenin. 293T cells, transfected with siUSP4 and HA‐Ub were treated with 10 μM MG132 for 4 h prior to harvest. The harvested cells were denatured by SDS and immunoprecipitated using anti‐β‐catenin antibody. The ubiquitinated β‐catenin was detected by SDS‐PAGE and immunoblotting. USP4 level in whole cell lysates was also examined by immunoblotting. (E) The effect of siUSP4 on β‐catenin stability. USP4 was knocked down using siUSP4 (+: 20 pM, ++: 50 pM) in 293T cells. After 48 h, USP4 and β‐catenin levels were analyzed by immunoblotting. α‐tubulin was used as a loading control. (F) The effect of cycloheximide and USP4 on β‐catenin stability. 293T cells were transfected with 60 pM siUSP4 and after 48 h treated with 100 μg/ml cycloheximide for the indicated times. Scrambled siRNA‐transfected cells were used as a control (NS). Immunoblots of endogenous USP4 and β‐catenin are shown. GAPDH was used as a loading control. The bar graph shows the band intensities of USP4 and β‐catenin. Each sample were normalized relative to the loading control.
