Figure 4.
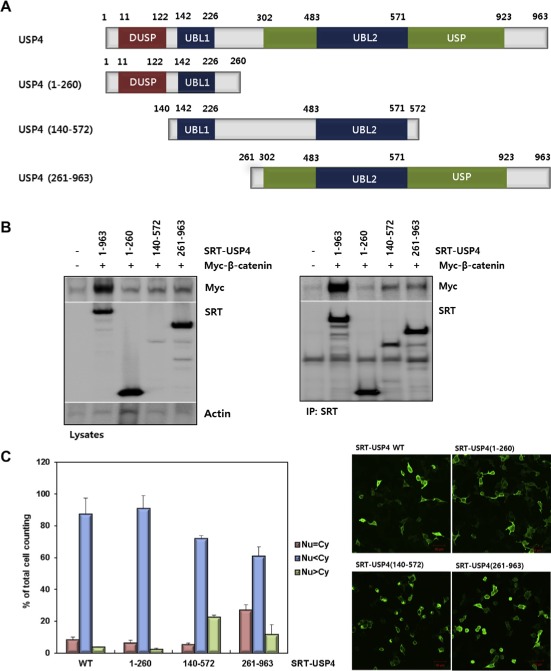
Domain mapping of USP4. (A) The domain structure of USP4 and design of the truncation mutants. Three constructs, USP4(1–260), USP4(140–572), and USP4(261–963), were drawn with residue numbers. The representative domains are also shown (DUSP, domain present in ubiquitin‐specific proteases; UBL, ubiquitin‐like domain; USP, ubiquitin specific proteases domain). (B) Interaction between the truncation mutants of USP4 and β‐catenin in 293T cells. Myc‐β‐catenin was co‐transfected with SRT‐tagged wild‐type (WT) or truncated USP4. Cells lysates were immunoprecipitated with anti‐SRT antibody. USP4 and β‐catenin levels in whole cell lysates and IP eluents were immunoblotted using the indicated antibodies. Actin was used as a loading control. (C) Subcellular localization of USP4 mutants. Each construct was transfected into 293T cells and their localization was quantified by counting the number of cells in three categories as described in Figure 3C (left). Localization of USP4 mutants were also visualized by immunostaining (right). Scale bars: 50 μm. (D) The effect of USP4 mutants on the TOP flash reporter activity. Myc‐β‐catenin was co‐expressed with the wild‐type or each truncation mutant in 293T cells for 48 h. TOP flash luciferase activity was estimated after normalization with pRL‐SV40 activity. (E) The effect of USP4 mutants on the expression of Cyclin D1. Myc‐β‐catenin and Cyclin D1 were immunoblotted with indicated antibodies (left). The band intensity of immunoblots was quantified and displayed as the relative amount to the control (right).
