Figure 3.
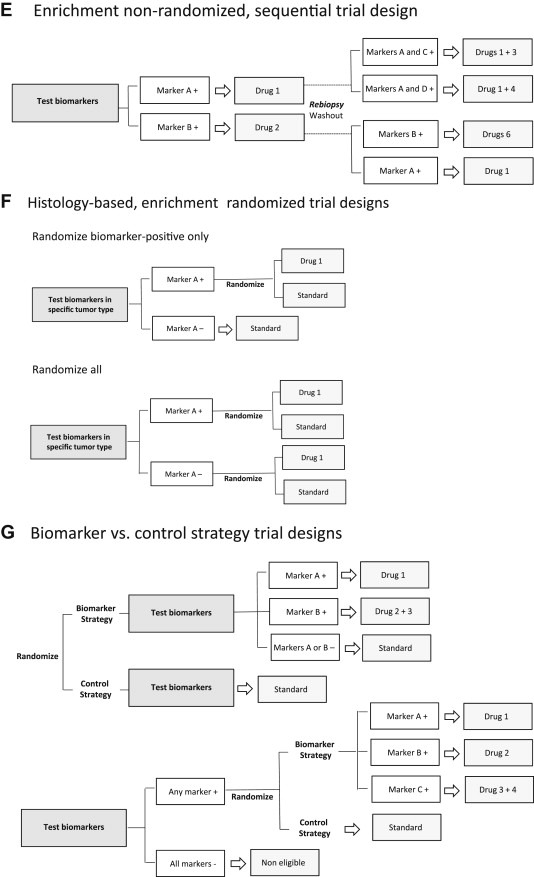
A. Histology‐agnostic, aberration‐specific trials (“Basket” design), patients receive a matched targeted therapy according to genomic profile irrespective of cancer type; B. Original “N‐of‐1” design with randomization, with analysis of the average effects of each drug across individuals; C: Modified “N‐of‐1” sequential design with each patient as his/her own control, comparing progression‐free survival on therapy guided by molecular aberration with that achieved on the regimen immediately preceding trial enrollment; D: Histology‐based, enrichment non‐randomized, hypothesis‐generating design; E: Enrichment non‐randomized sequential approach, with rebiopsies in the refractory setting guiding a second‐generation drug or a combination of targeted agents; F: Different randomized frameworks – to assess the predictive capacity of biomarkers with unknown clinical validity one needs to have the outcome of biomarker‐positive and ‐negative patients separately after experimental and control treatments, and to evaluate if a biomarker is prognostic, one needs to assess the outcome of biomarker‐positive and ‐negative patients on control treatment; G: Biomarker versus control strategy, with direct comparison of the outcomes of patients treated according to a biomarker‐based strategy with those assigned a control treatment.
