Figure 2.
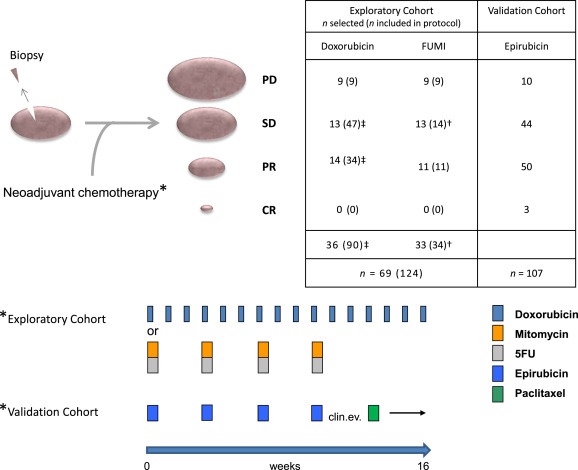
Study design and treatment regimens of included patients. All patients were diagnosed with locally advanced breast cancers and subject to biopsy for genetic analyses before commencement of neoadjuvant chemotherapy. Post therapy, all patients were evaluated for primary response to therapy before surgery (CR = Complete response, PR = Partial Response, SD = Stable Disease, PD = Progressive Disease, see Supplementary Information for details). In the exploratory cohort (n = 69), 36 of the patients were originally selected from the 90 patients enrolled in a prospective study assessing resistance to treatment with doxorubicin in locally advanced breast cancer (‡ selection of all patients with PD along with representative control groups of SD and PR). These patients received weekly doxorubicin for 16 weeks or until progressive disease was recorded. The remaining 33 patients were from a prospective study assessing resistance to mitomycin and 5‐fluorouracil (FUMI; † one patient was omitted due to lack of biological material). These patients received mitomycin and FUMI every third week (four cycles). Patients in the validation cohort received epirubicin every third week (four cycles) before clinical evaluation of response and change of therapy to paclitaxel every third week (four cycles) in case of an inferior response to the first line treatment. For further information regarding the cohorts, see Supplementary Information.
