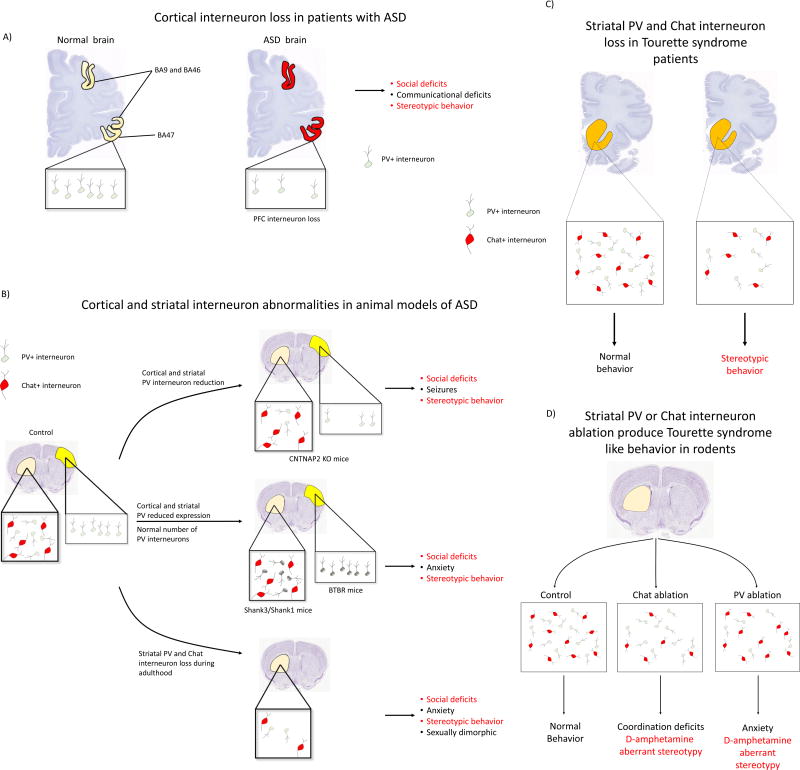
Figure 1.
INs alterations in ASD and TS, from humans to rodent models. A) Medial prefrontal cortex loss of PV+ INs in Broadmann areas (BA) BA46, BA47, and BA9 [62] has been found in postmortem studies in patients with ASD. B) Somatosensory cortices and striatum are affected by either PV interneuron death or PV lower levels of expression in rodent models of ASD. Different KO mice based on ASD genetic abnormalities found in ASD patients showed disruption in PV INs . Deletions in the shank3 and shank1 mice produces a reduction in the levels of PV expression without cell loss. In the CNTNAP2 KO mice, another well validated model of ASD, there is a reduction in the number of PV+ INs in the striatum and somatosensory cortex, where the main issue it seems to be migration impaired migration of progenitors. The idiopathic spontaneous inbred BTBR mice have a reduction in the expression of PV only in somatosensory cortices without alterations in other regions. Adulthood striatal ablation of PV and Chat INs showed to produces ASD behaviors like stereotypy and social deficits in a sexually dimorphic way, proposing a novel role of striatal INs in brain physiology. These different animals models of ASD shows a potential an exciting shared alteration that produces stereotypic behaviors and social deficits (in red): cortical and/or striatal INs abnormalities as a common denominator for ASD. C) Post mortem studies in TSpatients shows a significant reduction in the anumber of Chat and PV striatal INs. D) Striatal ablation of either PV or Chat INs mimics part of the core behaviors observed in TS like stereotypy. Single depletion of either type of these interneurons does not produce basal stereotypy, nevertheless this kind of behavior is strongly exacerbated after d-amphetamine administration.