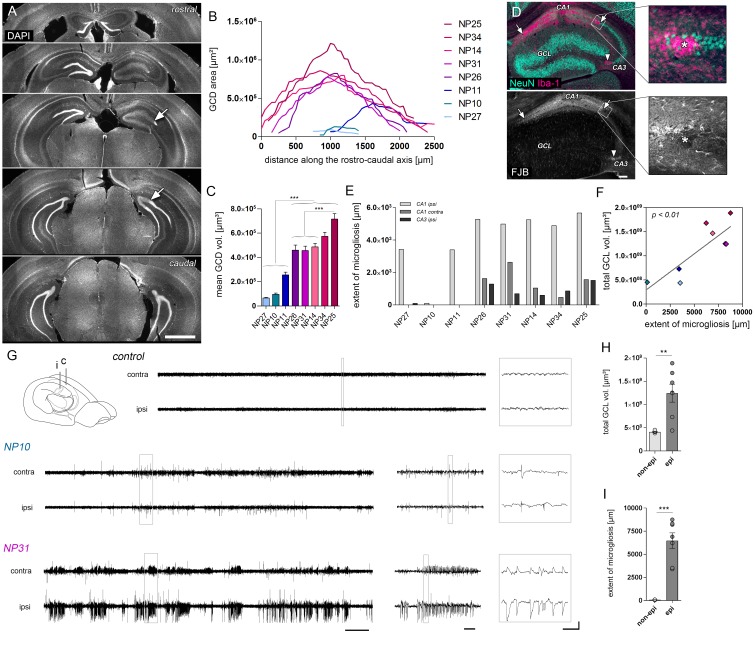
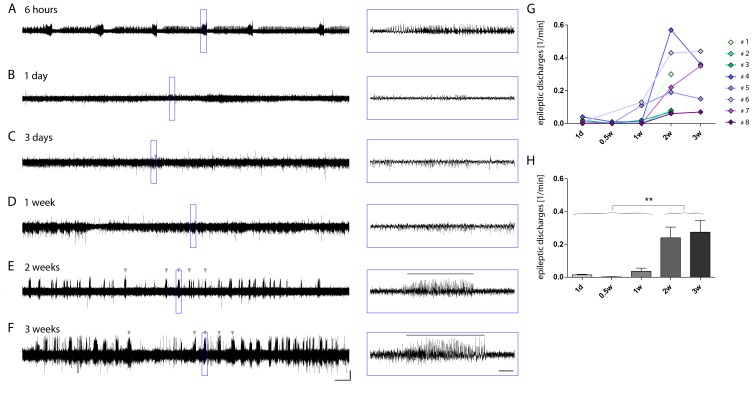
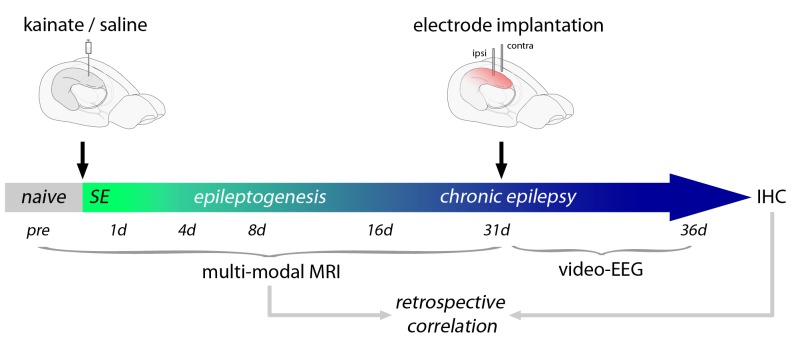
Figure 1. The severity of histological changes associated with HS varies in chronically epileptic mice.
(A) Representative DAPI-stained sections at different levels along the rostro-caudal hippocampal axis showing the extent of GCD (arrow indicates the transition to non-dispersed regions). (B) Corresponding quantitative analysis of the GCD area along the rostro-caudal axis, and (C) the calculated mean GCD volume from all analyzed sections tested for individual kainate-injected mice (one-way ANOVA, Bonferroni’s post-test; ***p<0.001; n = 8). (D) Representative photomicrographs of NeuN (turquoise; neurons) and Iba-1 (magenta; microglia) double immunostaining (upper panel) and Fluoro-Jade B (FJB) staining in consecutive sections. Clusters of amoeboid microglia are tightly associated with FJB-stained dying neurons (arrows and asterisks). (E) Quantitative analysis of cell death-associated microglial scarring in different regions (CA1 ipsi and contra; CA3 ipsi). (F) Regression analysis for the degree of GCD and the extent of microgliosis (summed for all regions) in kainate-injected mice (n = 8; Pearson’s correlation). Kainate-injected mice (NP10, NP11, NP14, NP26, NP27, NP31, NP34) are color-coded. Scale bars in A, 1 mm; in D, 200 µm. (G) Schematic of the mouse brain adapted from Witter and Amaral, 2004. Representative EEG traces of non-epileptic mice (controls or mice displaying only single epileptic spikes, NP10) and one example of an epileptic mouse displaying both epileptic spikes and paroxysmal discharges (NP31). Horizontal scale bars (left) 50 s, (middle) 5 s, (right) 0.5 s; vertical scale bar 2 mV. (H–I) Quantitative analysis of the total GCL volume (summed for all analyzed sections) and extent of microgliosis for epileptic (dark grey) and non-epileptic mice (light grey), respectively. Student’s t-test; **p<0.01, ***p<0.001; nnon-epi = 6, nepi = 7. All values are presented as the mean ± SEM.