Abstract
Study Objectives:
Several studies have suggested that rhinitis contributes to the pathogenesis of obstructive sleep apnea (OSA). We aimed to investigate the prevalence and influence of allergic rhinitis (AR) and non-allergic rhinitis (NAR) on severity of OSA.
Methods:
Two hundred forty patients with OSA confirmed by standardized polysomnography were assessed for presence of AR and NAR, using validated questionnaires and skin prick tests. Data comparison was carried out by using chi-square test, analysis of variance, and least significant difference test. Associations between severity of OSA and rhinitis, age, sex, and body mass index were assessed with ordinal logistic regression analysis.
Results:
The prevalence of AR and NAR among patients with OSA was 27.1% and 28.7%, respectively, with no significant differences in the severity of rhinitis. Ordinal logistic regression analysis showed AR and NAR were not the risk factors for severity of OSA. There were significant differences of polysomnography parameters in sleep efficiency (79.7 ± 2.0 versus 85.2 ± 1.4 between AR and NAR; 79.7 ± 2.0 versus 87.2 ± 1.4 between AR and no-rhinitis) and arousal index (36.8 ± 4.1 versus 24.7 ± 3.5 between AR and no-rhinitis). Patients with NAR had lower average arterial oxygen saturation (91.9 ± 0.6 versus 94.0 ± 0.6) and minimal arterial oxygen saturation (70.6 ± 1.7 versus 77.3 ± 1.8), compared with subjects categorized as no-rhinitis.
Conclusions:
This study suggests that despite a comparatively high prevalence in patients with OSA, the presence or severity of AR or NAR does not influence the severity of OSA; however, rhinitis may significantly disturb sleep in patients with OSA.
Citation:
Zheng M, Wang X, Ge S, Gu Y, Ding X, Zhang Y, Ye J, Zhang L. Allergic and non-allergic rhinitis are common in obstructive sleep apnea but not associated with disease severity. J Clin Sleep Med. 2017;13(8):959–966.
Keywords: allergic rhinitis, non-allergic rhinitis, obstructive sleep apnea, polysomnography
INTRODUCTION
Sleep deprivation has been shown to adversely affect health-related quality of life.1 Sleep-disordered breathing, a major cause of sleep deprivation, represents a large spectrum of abnormal conditions during sleep, ranging from simple or primary snoring to obstructive sleep apnea (OSA). OSA is characterized by repetitive obstruction of the upper airway often resulting in oxygen desaturation and arousals from sleep2,3 that affects approximately 13% of men and 6% of women among age 30 to 70 years.4 Patients with OSA often complain of restless sleep, nocturnal enuresis, excessive daytime somnolence, irritability, fatigue, and poor concentration.2,3 Risk factors in adults include aging, obesity, male sex, alcohol, menopause, cranio-facial dysmorphia, increased pharyngeal soft tissue and nasal obstruction.3,5,6 OSA has been shown to be associated with metabolic dysregulation and cardiovascular sequelae—such as diabetes mellitus, hypertension, congestive heart failure, and stroke3—and some studies have suggested that OSA may develop in patients with rhinitis experiencing frequent snoring followed by apneas.7,8
BRIEF SUMMARY
Current Knowledge/Study Rationale: Several studies have suggested that rhinitis contributes to the pathogenesis of obstructive sleep apnea. However, to date, few studies have investigated the prevalence of AR and NAR in patients with OSA.
Study Impact: The presence or severity of AR or NAR does not influence the severity of OSA; however, rhinitis may significantly disturb sleep in patients with OSA. As rhinitis may lead to significant changes in sleep patterns in patients with OSA, AR and NAR may be considered as symptoms potentiating, rather than risk-potentiating factors in the pathophysiology of OSA.
Rhinitis has been classified as allergic rhinitis (AR) or non-allergic rhinitis (NAR) based on a positive skin prick test (SPT) or presence of serum-specific immunoglobulin E. AR is a common upper airway disease affecting 10% to 20% of the world's population.9 The severity of AR is partly classified on the basis of its effect on sleep impairment10; patients with AR and nasal congestion are reported to be 1.8 times more likely to suffer from moderate to severe sleep-disordered breathing compared to patients without nasal congestion due to allergy.11 Indeed, one earlier study has suggested that in patients with seasonal AR, obstructive apneas may be longer and more frequent during a period of symptomatic nasal obstruction than when symptoms are absent.12 Compared to AR, the association between NAR, which affects more than 200 million in the world,13 and sleep-disordered breathing is less well established. However, some evidence suggests that patients with non-allergic rhinitis with eosinophilia syndrome (NARES) may suffer from more severe OSA, compared with patients without rhinitis.14 Furthermore, OSA may be more common in patients with NAR than AR.15 It has been reported that chronic nasal inflammation, such as NARES, might activate and augment neuronal reflex branches, like the so-called nasal-pulmonary reflex, causing alveolar hypoventilation, which resulted in an increase in hypopnea index.14,16
To date, comparatively few studies have investigated the prevalence of AR and NAR in patients with OSA. Thus, we tested the hypothesis that the prevalence of AR and NAR was significantly higher in patients with OSA and associated with the severity of OSA.
METHODS
Sample Size
Power Analysis and Sample Size (PASS) software (version 11, NCSS Statistical Software, Utah, United States) was used to calculate the number of enrolled patients with OSA in this survey, using an estimated prevalence of AR equal to 30% based on the prevalence of two previous epidemiological studies of individuals with OSA.17,18 As a result, the sample size of 226 produced a two-sided 90% confidence interval with a width equal to 0.1, when the sample proportion was 0.3. However, in order to reduce the probability of type 2 errors, more than 226 patients with OSA were expected to be enrolled in this study.
Patients
Subjects aged 18 years or older referred to the sleep monitoring center of the ear, nose, and throat department of Beijing TongRen Hospital from January 2010 to October 2011 for snoring and sleep apnea complaints were assessed for a diagnosis of OSA using polysomnography (PSG). Overall, 240 consecutive patients were found to be eligible and recruited to the study. The diagnosis and severity of OSA was defined according to the apnea-hypopnea index (AHI). Presence of OSA was defined as AHI > 5 events/h; mild OSA, AHI = 5–15 events/h; moderate OSA, AHI = 16–30 events/h; severe OSA, AHI > 30 events/h.19 Pregnant and nursing mothers were excluded, as were females planning to become pregnant in the near future. Similarly, patients with confirmed sinus diseases (such as nasal polyp, chronic sinusitis history, and deviation of nasal septum that seriously affects the ventilation status of the nasal cavity) and those with a history of nasal endoscopic surgery were excluded. Patients with OSA with any other limitations (eg, mental abnormality, hypophrenia, dyslexia, illiteracy) that might affect their ability to participate in the project were excluded.
Study Design
Eligible patients with OSA were examined by endoscopy and divided into three patient groups: AR, NAR, or no-rhinitis; based on skin prick test (SPT) results and the patients' responses to simple questionnaires comprising several questions regarding nasal symptoms, duration, and severity. These questionnaires were adapted from the well-documented and validated questionnaires used in the International Study of Asthma and Allergies in Childhood and the European Community Respiratory Health Survey studies, as we have described previously.20 Furthermore, the criteria used to diagnose AR were consistent with those of the ARIA (Allergic Rhinitis and Its Impact on Asthma) guidelines,10 and included a history of the typical symptoms of rhinorrhea, nasal congestion, sneezing, and/ or nasal itching during the past 12 months concomitantly with positive SPT to at least one aeroallergen. NAR was diagnosed similarly based on the history of symptoms, but with a negative SPT, whereas “no-rhinitis” was classified on the basis of the absence of any of these characteristic symptoms. The severity of both AR and NAR was further classified into four subtypes: mild intermittent, mild persistent, moderate-severe intermittent, and moderate-severe persistent, depending on the severity of symptoms and quality-of-life outcomes and self-reported duration of symptoms.21
The study was approved by the Ethics Review Committee of Beijing TongRen Hospital and all patients provided written informed consent prior to enrolment in the study.
Skin Prick Tests
All eligible participants with OSA were instructed to abstain from taking any antihistamines for at least 72 hours before the examination. SPTs were performed using the Allergopharma system (Allergopharma, Reinbeck, Germany) using a panel of 20 standardized allergen extracts (100,000 BU for Phazet and 10 HEP for Soluprick) comprising two seasonal and perennial subgroups. The seasonal panel consisted of extracts of two tree mixtures (tree I, tree II), cereals, mugwort, dandelion, ragweed, chenopodium album, humulus, Black locust, pine, and English plantain, and the perennial panel extracts of mixed animal hair, Blattella germanica, two mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), and five fungi (Curvularia lunata, Candida albicans, Penicillium notatum, Alternaria tenuis, and Aspergillus fumigatus). A positive (10 mg/mL histamine) and a negative (saline) control were also included.
The allergen extracts were applied 20 mm apart on the volar side of the forearm in a straight line from approximately 10 cm above the wrist and gently pricked into the skin. The skin reaction toward each allergen was observed after 15 minutes and the size of the reaction evaluated by tracing and measuring the perimeter of the wheal developed in response to any specific allergen. The largest and the smallest diameters (at the widest point and at the midpoint at 90°) of each wheal were measured and calculated as the mean wheal diameter. SPT reactivity was expressed as the mean allergen to histamine wheal ratio, and graded from 1+ to 4+. Thus, an allergen to histamine wheal ratio ≥ 0.5 for a positive SPT to an allergen was defined as a grade 2+ reaction.
Polysomnography and Related Demographic Information
Complete PSG was performed overnight in the sleep monitor center of Beijing TongRen Hospital, using the Alice 4 device (Healthdyne, Atlanta, Georgia, United States). Sleep and respiratory events were scored in each patient according to the principles and standard criteria of the American Academy of Sleep Medicine.22 Physiologic signals monitored included electroencephalograms (C3/A2, C4/A1), bilateral electro-oculograms, bipolar submental and tibialis anterior electromyograms, electrocardiograms, thoracic and abdominal movements, oronasal airflow (recorded by both a nasal pressure transducer and a thermal sensor) and arterial oxygen saturation. Demographic data, including age, sex, and body mass index (BMI; calculated as body weight [kg] divided by squared height [m2]) were recorded and the patients were classified into three categories according to their BMI values: normal weight (BMI ≤ 25 kg/m2), overweight (25 < BMI < 29 kg/m2), and obese (BMI ≥ 30 kg/m2).23 The PSG parameters were as follows: AHI; total sleep time; minimal arterial oxygen saturation (minimal SaO2); average arterial oxygen saturation during sleep (average SaO2); time spent with an oxygen saturation below 90% (time of SaO2 < 90%); sleep efficiency (%), oxygen desaturation index; arousal index; REM sleep time; stage 1 sleep time (S1); stage 2 sleep time (S2); stage delta sleep time (delta). Furthermore, apnea was defined as > 90% reduction in airflow for more than 10 seconds whereas hypopnea was defined as > 50% decrease in airflow for more than 10 seconds or < 50% decrease, but with 4% decrease in oxygen saturation.
Statistical Analysis
Statistical analyses were conducted using the SPSS 17.0 software for Windows (SPSS Inc., Chicago, Illinois, United States). Continuous study variables were expressed as mean ± standard error. Proportions between groups were tested using the chi-square test (χ2). Comparison among the three groups was carried out using one-way analysis of variance for normally distributed variables, in which the multiple comparisons were analyzed by employing least significant difference (LSD) post hoc test. The ordinal logistic regression was performed to assess the relationship of the severity of OSA with the potential risk factors such as age, sex, BMI, and the diagnosis of rhinitis. All tests were based on two-tailed tests using a significance level of P < .05.
RESULTS
Demographic Characteristics and Grading
Overall, 240 patients with OSA were recruited to the study; 217 (90.4%) were male and 23 (9.6%) female (Table 1). Approximately one-third of participants (32.1%) were between 38 and 47 years of age, and one-fourth (25%) between 28 and 37 years of age. Furthermore, nearly 75% of the patients received a diagnosis of moderate to severe OSA.
Table 1.
Demographic characteristics and OSA severity.
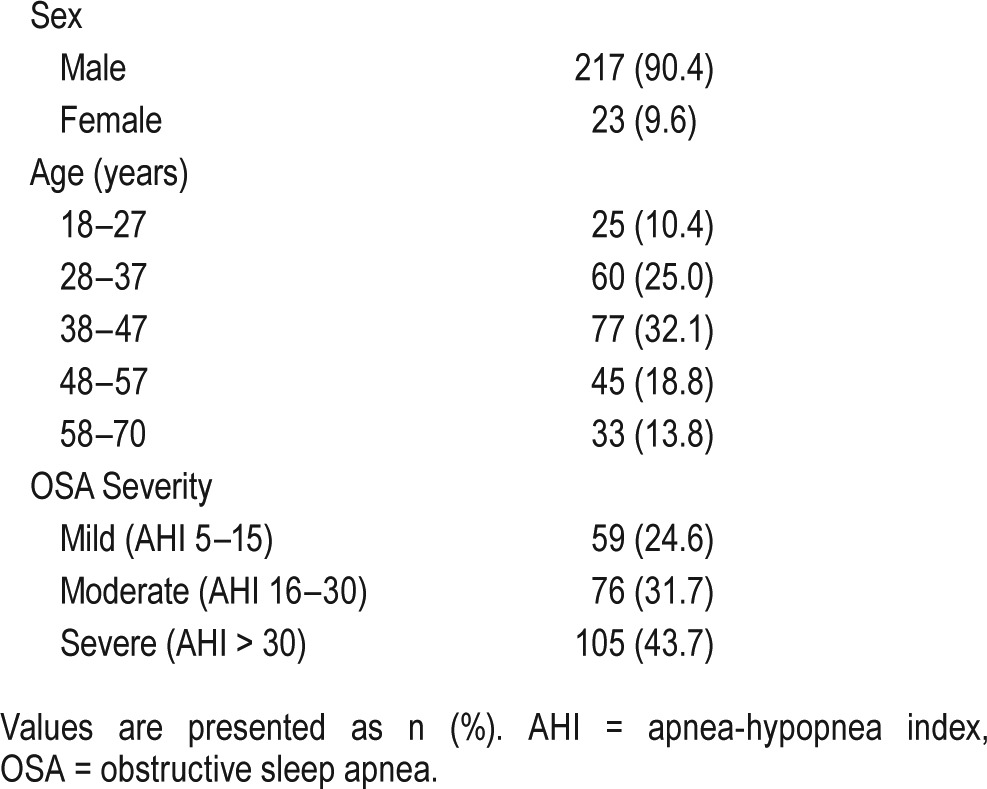
Prevalence of AR, NAR, and No-Rhinitis in Patients With OSA
One hundred thirty-four of the 240 patients with OSA received a diagnosis of rhinitis; 65 (27.1%) had AR and 69 had NAR. The remaining 106 patients (44.2%) did not show any symptoms of rhinitis. Moreover, 7.5% and 5.4% of patients with AR and NAR, respectively, demonstrated mild OSA severity, whereas 13.3% and 12.5% of patients with AR and NAR, respectively, demonstrated high OSA severity. Statistical analysis showed that there were nonsignificant differences in various OSA severity (total P = .357) with respect to the proportion of AR, NAR, and no-rhinitis (Table 2).
Table 2.
The prevalence of AR or NAR in patients with different severity of OSA.
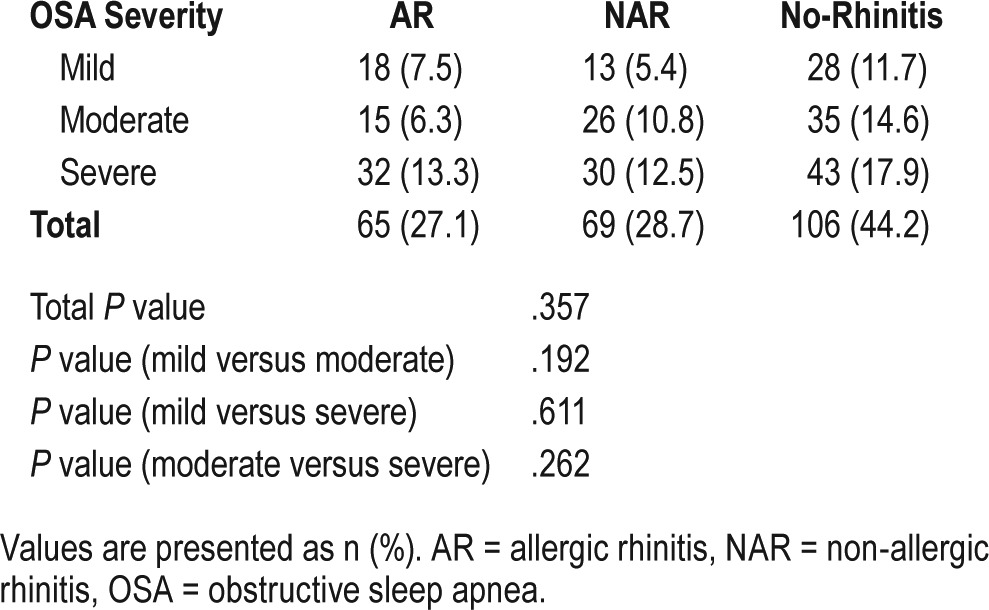
Subdivision of Rhinitis in Patients With OSA
Table 3 shows the prevalence of mild and moderate-severe intermittent or persistent rhinitis in patients with different OSA severity. Analysis using the chi-square test demonstrated that the proportions of patients with different rhinitis subtypes in the three OSA subgroups were significantly different (P = .049). However, further analysis demonstrated that the proportions of patients with different rhinitis subtypes were not significantly different in patients with mild OSA compared to patients with moderate OSA (P = .311) or compared to patients with severe OSA (P = .022); or between patients with moderate and severe OSA (P = .228).
Table 3.
The prevalence of mild and moderate-severe intermittent or persistent rhinitis in patients with different OSA severity.
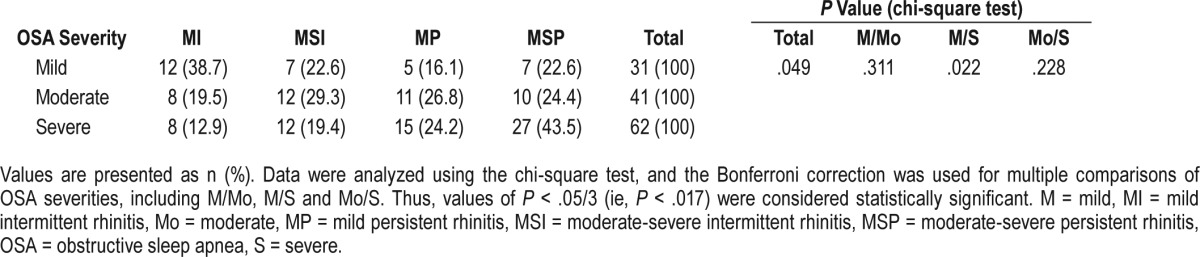
BMI and PSG Parameters in Patients With OSA and Differential Diagnosis of Rhinitis
Table 4 shows the results for BMI and the PSG parameters assessed. The BMI values were not significantly different between patients with OSA with or without a diagnosis of rhinitis. Comparison of PSG parameters among the three groups of patients with OSA demonstrated that although total sleep time was similar for all three groups, the quality of sleep was more disrupted in patients with AR, as evidenced by significantly lower sleep efficiency in patients with AR compared to patients with NAR (79.7 ± 2.0 versus 85.2 ± 1.4, P = .013) and patients categorized as no-rhinitis (79.7 ± 2.0 versus 87.2 ± 1.4, P = .002). Similarly, the results of the LSD test also showed that the arousal index in patients with AR was significantly higher compared to those categorized as no-rhinitis (36.8 ± 4.1 versus 24.7 ± 3.5, P = .048), but not compared to patients with NAR. In contrast, according to the LSD tests, oxygen saturation was significantly lower in patients with NAR compared to those categorized as no-rhinitis, as evidenced by both the average SaO2 saturation (91.9 ± 0.6 versus 94.0 ± 0.6, P = .016) and the minimal SaO2 saturation (70.6 ± 1.7 versus 77.3 ± 1.8, P = .017). No significant differences were observed between the three groups for REM sleep and various stages of slow wave sleep, respiratory events, or the mean AHI value.
Table 4.
Comparison of body mass index and PSG parameters between patients with OSA and AR, NAR, and no-rhinitis by analysis of variance and least significant difference test.
Ordinal Logistic Regression Analysis
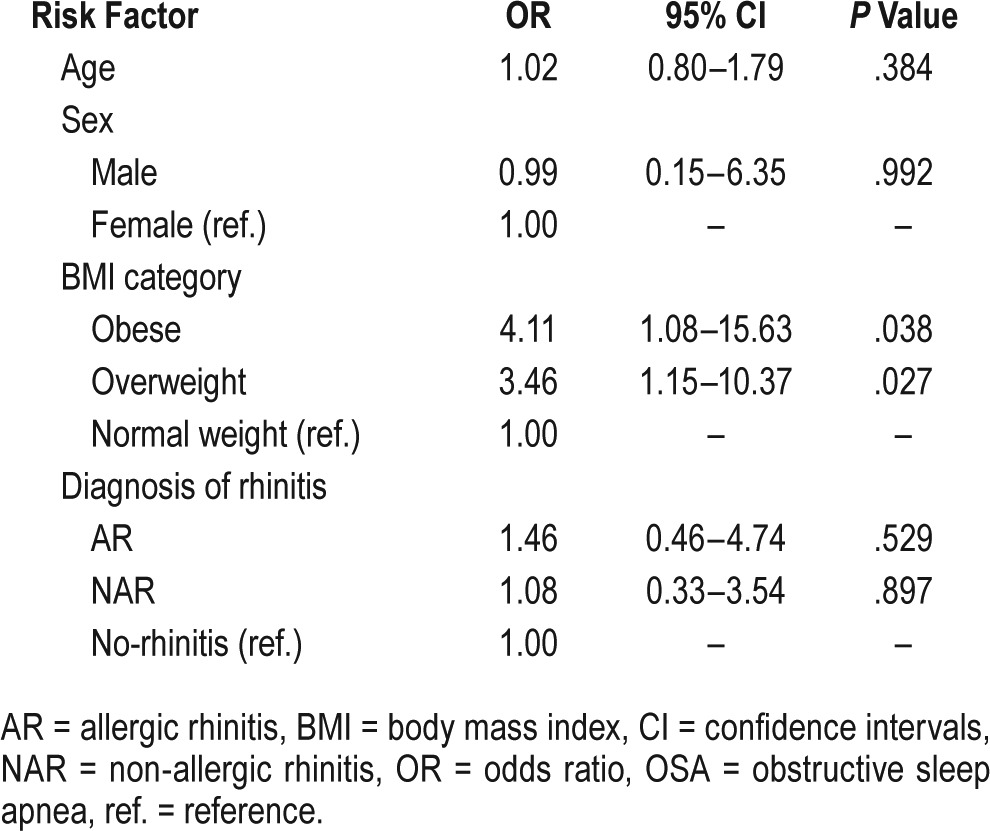
Ordinal logistic regression analysis of potential risk factors associated with the severity of OSA showed that there was a significant relationship between the increase in BMI category and severity of OSA. Using the normal weight as the reference, obese and overweight significantly increased the risk of having more severe OSA (odds ratio [OR] = 4.11, 95% confidence interval = 1.08–15.63; OR = 3.46, 95% confidence in -terval = 1.15–10.37, respectively). In contrast, no significant association was demonstrated between the severity of OSA and age, sex, or the presence of rhinitis (Table 5).
Table 5.
Ordinal logistic regression models of potential risk factors associated with severity of OSA.
Characteristic of Positive Aeroallergens in Patients With AR and OSA
Among the 65 patients with AR and OSA, 32 (13.3%) were present in severe OSA group, 19 (7.9%) in the moderate severity group, and 14 (5.8%) in the mild severity group. Based on the sensitization to seasonal and perennial aeroallergens, the 65 patients with AR were divided into subgroups with three sensitization patterns: 14 patients (21.5%) sensitized to only seasonal allergens, 19 patients (29.2%) sensitized to both seasonal and perennial allergens, and 32 patients (49.2%) sensitized to only perennial allergens. Assessment of the number of patients with a particular sensitization pattern with OSA severity demonstrated that the largest number of patients from each sensitization group were present in the group with severe OSA; with 14 patients (21.5%) sensitized to perennial allergens comprising the largest group (Figure 1). In contrast, patients with mild OSA comprised the smallest number of patients among each sensitization group.
Figure 1. Percentages of different positive aeroallergens in OSA subgroups of different severity.
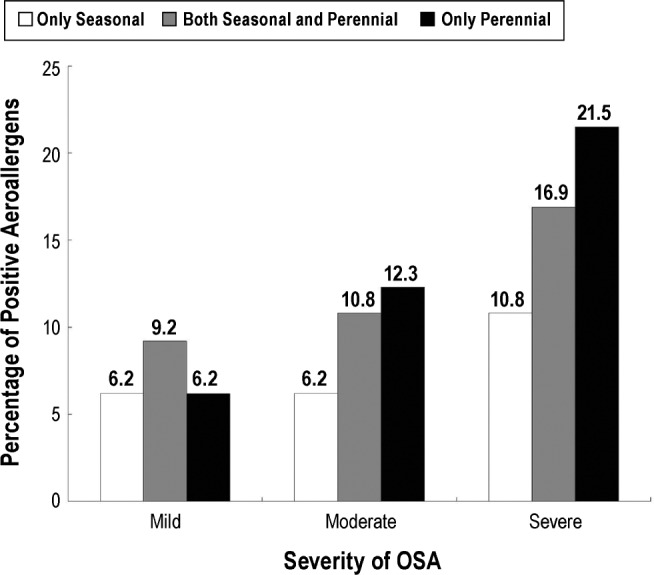
OSA = obstructive sleep apnea.
DISCUSSION
Using validated questionnaires, SPT, and PSG, we identified higher prevalence of AR (27.1%) and NAR (28.1%) among patients with OSA, than overall prevalence of AR and NAR at 17.6% and 16.4%, respectively, in the general population surveyed in 18 major cities of China.24 Currently, rhinitis has been identified as a potential risk factor in sleep-disordered breathing, including OSA.25 Several studies have focused on the potential immunologic association between sleep disruption and AR. One study in a group of four allergic and four non-allergic subjects demonstrated that the serum proallergic cytokines (IL-1β, IL-4, and IL-10) were higher in allergic subjects, and correlated with increased latency to the onset of REM sleep and shorter time of REM sleep,26 suggesting that allergic subjects may be more prone to drowsiness or insomnia than non-allergic subjects. Moreover, recent studies have reported that the Th17/Treg ratio in peripheral blood is upregulated in children with OSA and AR to a greater extent than in children without AR, suggesting that AR could possibly promote the development of OSA.27 Although the precise underlying patho-physiological mechanisms are not currently clear, it has been suggested that increased nasal resistance associated with AR and NAR, which results in negative pressure in the pharynx, predisposes the nose to additional upper airway collapse and exacerbates its effects during sleep because a subject is lying in a horizontal position.25,28–30 Similarly, Jiang and colleagues31 have reported that patients with chronic rhinosinusitis who needed sinus surgery had a very high prevalence of OSA (64.7%), although there was no correlation with the severity of rhinosinusitis. Indeed, Blakley and colleagues have shown that nasal resistance in patients with OSA is higher than in control subjects.32 The finding that habitual snoring in children is associated with the number of rhinitis symptoms and a previous diagnosis of rhinitis and allergic sensitization33,34 suggests that rhinitis symptoms other than nasal obstruction also are likely to play a role in the pathogenesis of sleep-disordered breathing.
To our knowledge, the current study is the first epidemio-logical survey to determine the prevalence of AR and NAR in patients with OSA. However, the findings of the current study are somewhat limited because the actual differences in the prevalence of AR and NAR in patients with OSA was not directly compared to that of controls without OSA, but to that of historical controls. Thus, it is possible that a lack of controls without OSA in this study could potentially have underestimated the prevalence and role of both AR and NAR in the pathogenesis of OSA. Despite this limitation the findings from the current study still provide useful information in the context of other studies in this field of investigation. One study by Canova and colleagues35 reported a prevalence of 11% perennial AR in patients with OSA. Furthermore, more patients with OSA were sensitized to perennial allergens; particularly to house dust mite (23.6 versus 4.5%) and dog dander (18.0 versus 4.5%); than patients with COPD. Among the 65 patients with OSA and AR investigated in the present study, 32 (49.2%) showed SPT positivity to only perennial allergens and 19 (29.2%) to both seasonal and perennial allergens, indicating that 21.25 % of the total study population of 240 patients with OSA were sensitized to perennial allergens. It is possible that the higher sensitization to perennial allergens noted in our study compared to that of Canova and colleagues35 was a result of our study using nine perennial allergens in the SPT to avoid underestimating AR, compared to five perennial allergens used by Canova and colleagues.35 A possible reason for higher prevalence of AR among patients with OSA is that obese subjects had a higher frequency of specific immunoglobulin E positivity than nonobese subjects.36 Moreover, the sympathetic activation in patients with OSA induced by periodic hypoxemia could increase the release of catecholamine, which might lead to T-helper 2 mediated allergic inflammation.37
A recent review of the effect of AR on sleep disruption has indicated that the degree of sleep impairment and associated symptoms appear to be directly related to the severity of the AR.38 A similar finding was also observed in our study. Among the 134 patients in whom rhinitis (AR and NAR) was diagnosed, only 7 patients with mild OSA had a diagnosis of moderate-severe persistent rhinitis, whereas 27 patients with severe OSA had moderate-severe persistent rhinitis. However, only 8 patients with severe OSA had mild intermittent rhinitis, and 12 patients with mild OSA had mild intermittent rhinitis.
The subgroups of patients with OSA were not significantly different with respect to the proportions of subjects with different types of rhinitis, and ordinal logistic regression analysis showed that presence of rhinitis, age, and sex were not risk factors for severity of OSA. Although Virkkula and colleagues39 have reported increased nasal resistance to be an independent predictor of increased AHI in nonobese individuals, this observation does not appear to be supported by several other studies using PSG in rhinitis. The Wisconsin Sleep Cohort Study involving 911 patients showed a nonsignificant increase in the AHI when nasal obstruction was present.11 Similarly, a controlled clinical trial comparing changes in sleep parameters such as apnea index, hypopnea index, and AHI in a group of patients with seasonal AR versus a group of healthy volunteers undergoing two consecutive nights of PSG before and during pollen season demonstrated minimal and nonsignificant changes between the two groups for all parameters.40 Another study by Koutsourelakis and colleagues41 also showed that AHI in 49 patients with OSA remained unchanged after nasal surgery, despite decreased nasal resistance. It is possible that rhinitis may have a minor role in increasing upper airway collapsibility and increased nasal congestion may contribute to sleep disruption via increased negative intrathoracic pressure swings as reflected by worse sleep efficiency detected in this study. It is also possible that the lack of an association between severity of OSA and rhinitis could be attributable to the heterogeneity of OSA.42 It is now recognized that there are at least four phenotypes in the pathogenesis of OSA, including collapsible upper airway, ineffective upper airway dilator muscles, low respiratory arousal threshold, and unstable ventilator control.43 These phenotypes are distinct among patients with OSA and therefore, rhinitis may be a contributor to OSA pathogenesis for patients with specific phenotypes, but not others. Thus, a comprehensive understanding of multiple causes of OSA should help to determine novel therapeutic targets and to develop novel therapies accordingly.
BMI was shown to be associated with severity of OSA in the current study; with obese and overweight significantly increasing the risk of having more severe OSA (OR = 4.11 and 3.46, respectively). It has been widely recognized that patients with OSA have greater body weight than the general population, and increased body weight plays an important role in the development of OSA. Namyslowski and colleagues44 have reported that there is a significant relationship between the increase in BMI and sleep parameters in obese subjects. Quintas and colleagues45 have demonstrated that although 64.28% of patients with OSA and normal weight had a mild disease, 41.4% of overweight patients had moderate OSA and 57.9% of obese patients had severe OSA.
The current study has also demonstrated significant differences in lower sleep efficiency between AR and NAR (79.7 ± 2.0 versus 85.2 ± 1.4) and between AR and no-rhinitis (79.7 ± 2.0 versus 87.2 ± 1.4). Furthermore, patients with AR had higher arousal index compared with those categorized as no-rhinitis (36.8 ± 4.1 versus 24.7 ± 3.5). A previous study also noted that patients with AR experience an approximate tenfold increase in the number of microarousals during sleep.46 Recently, a study investigating sleep impairment using PSG in patients with persistent AR showed that there were statistically significant, though clinically modest, differences between patients with persistent AR and healthy controls in most PSG parameters, including sleep efficiency, arousal index, average SaO2, lowest SaO2, time spent with a saturation below 90%, and snoring time.47 However, AHI was similar for the two groups. Interestingly, the current study has demonstrated that compared to patients with OSA and without rhinitis, patients with NAR, rather than with AR, had significantly lower average SaO2 saturation (91.9 ± 0.6 versus 94.0 ± 0.6) and minimal SaO2 saturation (70.6 ± 1.7 versus 77.3 ± 1.8). A possible explanation for this difference between patients with NAR and AR is that patients with NAR had more perennial, persistent, and moderate-severe rhinitis symptoms, whereas patients with AR had more seasonal, intermittent, and mild rhinitis symptoms.7,48 Our findings, however, are in accordance with those of Kramer and colleagues,14 who demonstrated that patients with OSA and NARES revealed significantly impaired PSG parameters including hypopnea index, and average and minimal SaO2 saturation, compared with patients without nasal inflammation.
CONCLUSIONS
In summary, this study suggests that despite a comparatively high prevalence in patients with OSA, the presence or severity of AR or NAR does not appear to influence the severity of OSA. Because rhinitis may lead to significant changes in sleep patterns in patients with OSA, AR and NAR may be considered as symptoms potentiating, rather than risk-potentiating factors in the pathophysiology of OSA. However, this needs to be confirmed in further studies directly comparing prevalence of AR and NAR in both patients with OSA and without OSA, as suitable controls. A lack of a control group without OSA in this study could potentially have underestimated the role of both AR and NAR in the pathogenesis of OSA.
DISCLOSURE STATEMENT
All the authors declare that they have seen and approved the manuscript and they have no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants from Merck & Co., Inc. (P05537), the program for Changjiang scholars and innovative research team (IRT13082), the national natural science funds (81420108009 and 81630023), Beijing natural science foundation (7131006), the 12th five-year science and technology support project (2014BAI07B04), the Capital health research and development program (2011-1017-06), and Beijing health bureau program for high-level talents (2009-2-007, 2011-3-039 and 2011-3-043).
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AHWR
allergen to histamine wheal ratio
- AR
allergic rhinitis
- BMI
body mass index
- CRS
chronic rhinosinusitis
- NAR
non-allergic rhinitis
- NARES
non-allergic rhinitis with eosinophilia syndrome
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SPT
skin prick test
- SWS
slow wave sleep
- TST
total sleep time
REFERENCES
- 1.Klumpers UM, Veltman DJ, van Tol MJ, et al. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. Plos One. 2015;10(1):e0116906. doi: 10.1371/journal.pone.0116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 3.Gharibeh T, Mehra R. Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep. 2010;2:233–255. doi: 10.2147/NSS.S6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stradling JR. Sleep-related breathing disorders. 1. Obstructive sleep apnoea: definitions, epidemiology, and natural history. Thorax. 1995;50(6):683–689. doi: 10.1136/thx.50.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lofaso F, Coste A, d'Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16(4):639–643. doi: 10.1034/j.1399-3003.2000.16d12.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalpaklioglu FA, Kavut AB, Ekici M. Allergic and nonallergic rhinitis: the threat for obstructive sleep apnea. Ann Allergy Asthma Immunol. 2009;103(1):20–25. doi: 10.1016/S1081-1206(10)60138-X. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Kushida CA. Nasal obstruction in sleep-disordered breathing. Otolaryngol Clin North Am. 2003;36(6):437–460. doi: 10.1016/s0030-6665(02)00175-5. [DOI] [PubMed] [Google Scholar]
- 9.Brozek JL, Bousquet J, Baena-cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 Revision. J Allergy Clin Immunol. 2010;126(3):466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Van Cauwenberge P, Khaltaev N Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep disordered breathing. J Allergy Clin Immunol. 1997;99(2):S757–S762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126(4):625–628. doi: 10.1164/arrd.1982.126.4.625. [DOI] [PubMed] [Google Scholar]
- 13.Kaliner MA. Classification of nonallergic rhinitis syndromes with a focus on vasomotor rhinitis, proposed to be known henceforth as nonallergic rhinopathy. World Allergy Organ J. 2009;2(6):98–101. doi: 10.1097/WOX.0b013e3181a9d55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer MF, Chaux R, Fintelmann R, Rasp G. NARES: a risk factor for obstructive sleep apnea? Am J Otolaryngol. 2004;25(3):173–177. doi: 10.1016/j.amjoto.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Vichyanond P, Suratannon C, Lertbunnaphong P, Jirapongsananuruk O, Visitsunthorn N. Clinical characteristics of children with non-allergic rhinitis vs with allergic rhinitis. Asian Pac J Allergy Immunol. 2010;28(4):270–274. [PubMed] [Google Scholar]
- 16.Papsidero MJ. The role of nasal obstruction in obstructive sleep apnea syndrome. Int Arch Allergy Immunol. 1993;72(1):82–84. [PubMed] [Google Scholar]
- 17.Shadan FF, Jalowayski AA, Fahrenholz J, Kline LE, Dawson A. Nasal cytology: a marker of clinically silent inflammation in patients with obstructive sleep apnea and a predictor of noncompliance with nasal CPAP therapy. J Clin Sleep Med. 2005;1(3):266–270. [PubMed] [Google Scholar]
- 18.Gui A, Cinar F, Evren C, Uğur MB, Sarikaya S. The prevalence of allergic rhinitis in patients with simple snoring and obstructive sleep apnea syndrome. Kulak Burun Boqaz Ihtis Derq. 2011;21(2):70–75. doi: 10.5606/kbbihtisas.2011.002. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.Zheng M, Wang X, Bo M, et al. Prevalence of allergic rhinitis among adults in urban and rural areas of China: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015;7(2):138–157. doi: 10.4168/aair.2015.7.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molgaard E, Thomsen SF, Lund T, Pedersen L, Nolte H, Backer V. Differences between allergic and nonallergic rhinitis in a large sample of adolescents and adults. Allergy. 2007;62(9):1033–1037. doi: 10.1111/j.1398-9995.2007.01355.x. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 23.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler M, Bloch KE, Stradling JR. The role of the nose in the pathogenesis of obstructive sleep aponea and snoring. Eur Respir J. 2007;30(6):1208–1215. doi: 10.1183/09031936.00032007. [DOI] [PubMed] [Google Scholar]
- 26.Krouse HJ, Davis JE, Krouse JH. Immune mediators in allergic rhinitis and sleep. Otolaryngol Head Neck Surg. 2002;126(6):607–613. doi: 10.1067/mhn.2002.125300. [DOI] [PubMed] [Google Scholar]
- 27.Ni K, Zhao LM, Wu JL, Chen W, Yang HY, Li XY. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2015;79(9):1448–1454. doi: 10.1016/j.ijporl.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick M, Driver H, Chatha N, Voduc N, Girard AM. Partitioning of inhaled ventilation between the nasal and oral routes during sleep in no-rhinitis subjects. J Appl Physiol. 2003;94(3):883–890. doi: 10.1152/japplphysiol.00658.2002. [DOI] [PubMed] [Google Scholar]
- 29.Park S. Flow-regulatory function of upper airway in health and disease: a unified pathogenetic view of sleep-disordered breathing. Lung. 1993;64(6):311–333. doi: 10.1007/BF00165698. [DOI] [PubMed] [Google Scholar]
- 30.Rundcrant H. Posturnal variations of nasal patency. Acta Otolaryngol. 1969;68(5):435–443. doi: 10.3109/00016486909121582. [DOI] [PubMed] [Google Scholar]
- 31.Jiang RS, Liang KL, Hsin CH, Su MC. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology. 2016;54(1):75–79. doi: 10.4193/Rhino15.204. [DOI] [PubMed] [Google Scholar]
- 32.Blakley B, Mahowald M. Nasal resistance and sleep apnea. Laryngoscope. 1987;97(6):752–754. doi: 10.1288/00005537-198706000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Tafur A, Cherrez-Ojeda I, Patino C, et al. Rhinitis symptoms and habitual snoring in Ecuadorian children. Sleep Med. 2009;10(9):1035–1039. doi: 10.1016/j.sleep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 34.McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111(1):170–173. doi: 10.1378/chest.111.1.170. [DOI] [PubMed] [Google Scholar]
- 35.Canova CR, Downs SH, Knoblauch A, Andersson M, Tamm, Leuppi JD. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration. 2004;71(2):138–143. doi: 10.1159/000076674. [DOI] [PubMed] [Google Scholar]
- 36.Vieira VJ, Ronan AM, Windt MR, Tagliaferro AR. Elevated atopy in healthy obese women. Am J Clin Nutr. 2005;82(3):504–509. doi: 10.1093/ajcn.82.3.504. [DOI] [PubMed] [Google Scholar]
- 37.Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinal Metab. 2002;87(9):4245–4251. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- 38.Soose RJ. Role of allergy in sleep-disordered breathing. Otolaryngol Clin N Am. 2011;44(3):625–635. doi: 10.1016/j.otc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Virkkula P, Hurmerinta K, Loytonen M, et al. Posturnal cephalometric analysis and nasal resistance in sleep-disordered breathing. Laryngoscope. 2003;113(7):1166–1174. doi: 10.1097/00005537-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol. 2004;113(4):663–668. doi: 10.1016/j.jaci.2003.12.589. [DOI] [PubMed] [Google Scholar]
- 41.Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008;31(1):110–117. doi: 10.1183/09031936.00087607. [DOI] [PubMed] [Google Scholar]
- 42.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea: new pathways for targeted therapy. Sleep Med Rev. 2016 Dec 18; doi: 10.1016/j.smrv.2016.12.003. doi: 10.1016/j.smrv.2016.12.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Namyslowski G, Scierski W, Mrowka-kata K, Kawecka I, Kawecki D, Czecior E. Sleep study in patients with overweight and obesity. J Physiol Pharmacol. 2005;56(Suppl.6):59–65. [PubMed] [Google Scholar]
- 45.Dacal Quintas R, Tumbeiro Novoa M, Alves Pérez MT, Santalla Martinez ML, Acuña Fernández A, Marcos Velázquez P. Obsturctive sleep apnea in normal weight patients: characteristics and comparison with overweight and obese patients. Archivos de Bronconeumologia. 2013;49(12):513–517. doi: 10.1016/j.arbres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Lavie P, Gertner R, Zomer J, Podoshin L. Breathing disorders in sleep associated with “microarousals” in patients with allergic rhinitis. Acta Otolaryngol. 1981;92(5-6):529–533. doi: 10.3109/00016488109133292. [DOI] [PubMed] [Google Scholar]
- 47.Meng J, Xuan J, Qiao X, et al. Assessment of sleep impairment in persistent allergic rhinitis patients using polysomnography. Int Arch Allergy Immunol. 2011;155(1):57–62. doi: 10.1159/000317244. [DOI] [PubMed] [Google Scholar]
- 48.Settipane RA. Demographics and epidemiology of allergic and nonallergic rhinitis. Allergy Asthma Proc. 2001;22(4):185–189. [PubMed] [Google Scholar]


