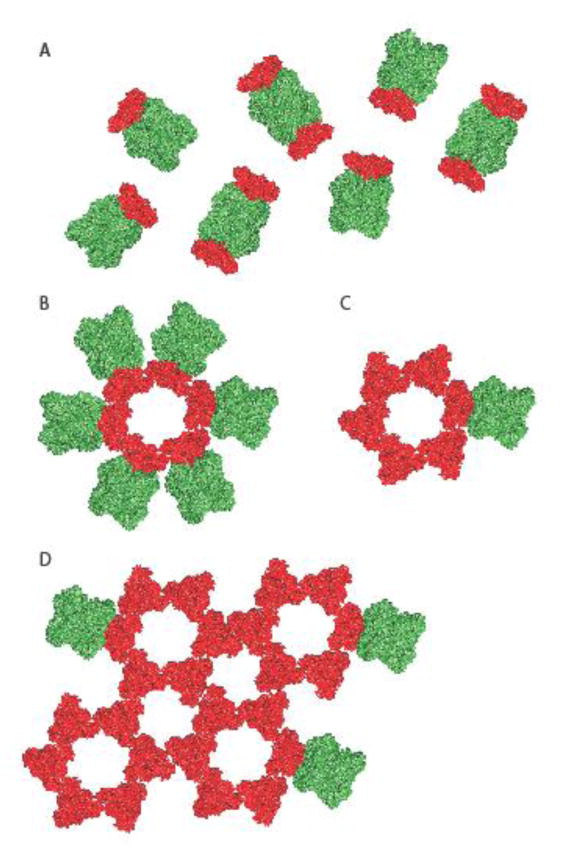
Figure 3.
Models of alternative spatial organizations of the paramyxovirus glycoproteins on the virion surface. A) The overall organization of the paramyxovirus glycoproteins was thought to be random with an undetermined relative stoichiometry of individual fusion protein trimers (red) and attachment protein tetramers (green). B, C, D) In a recent study [25], an arrangement of NiV F protein trimers into hexamers of trimers and higher order complexes was proposed. Schematics of hexameric F trimer arrangements in contact with one (A) or multiple (B) attachment protein tetramers and higher order F assemblies consisting of interacting hexamers of trimers (C). Different hypothetical contacts of the F assemblies with attachment protein tetramers are shown, but the stoichiometry and positioning of the attachment protein oligomers relative to the F protein complexes has not yet been defined.