Abstract
Feelings of hunger carry a negative-valence (emotion) signal that appears to be conveyed through agouti-related peptide (AgRP) neurons in the hypothalamic arcuate nucleus. The circulating hunger hormone, ghrelin, activates these neurons although it remains unclear whether it also carries a negative-valence signal. Given that ghrelin also activates pathways in the midbrain that are important for reward, it remains possible that ghrelin could act as a positive reinforcer and hence, carry a positive-valence signal. Here we used condition preference/avoidance tests to explore the reinforcing/aversive properties of ghrelin, delivered by intracerebroventricular (ICV) injection (2 µg/injection once a day for 4 days). We found that ICV ghrelin produces conditioned avoidance, both in a conditioned place preference/avoidance test (CPP/CPA, in which the animals avoid a chamber previously paired to ghrelin injection) and in a conditioned flavor preference/avoidance test (CFP/CFA, in which the animals consume/avoid a taste previously paired to ghrelin injection). These effects of ghrelin to induce a CPA were observed when conditioning to ghrelin occurred in the absence or presence of food. We did not find evidence, however, that brain ghrelin delivery to rats induces malaise (in the pica test). Our data indicate that ICV ghrelin carries a negative-valence signal consistent with its role as a circulating hunger hormone and with its effects to activate AgRP neurones.
Keywords: Hunger, AgRP, Valence, Condition place preference, Gut-brain signaling
1. Introduction
Recent studies suggest that appetitive and food seeking behaviors that occur when hungry and that precede consumption can be driven by unpleasant negative-emotion/valence signals, and that the motivation to seek food and the initiation of its consumption is generated by the learned alleviation of negative valence that occurs once the food has been eaten (Betley et al., 2015, Sternson and Eiselt, 2016). One key neuronal substrate transmitting this negative-valence signal is the agouti-related peptide (AgRP) neurons in the arcuate nucleus (ARC) of the hypothalamus (Betley et al., 2015). AgRP neurons (that, in addition, contain neuropeptide Y (NPY) and gamma aminobutyric acid (GABA)) are an important component in the pathways controlling homeostatic feeding; they receive information about peripheral energy status through endocrine signals such as leptin or ghrelin (that inhibit or activate these cells, respectively) (Cowley et al., 2003, Takahashi and Cone, 2005). The activation of AgRP neurons increases food consumption, while inhibition or ablation suppresses feeding (Aponte et al., 2011, Atasoy et al., 2012, Krashes et al., 2011, Luquet et al., 2005).
The circulating, stomach-derived, hunger hormone ghrelin is secreted preprandially (Cummings et al., 2001). Ghrelin receptor agonists increase food intake and activate ARC AgRP/NPY neurons (Cowley et al., 2003, Dickson and Luckman, 1997, Lawrence et al., 2002). In mice that lack AgRP, ghrelin fails to increase food intake suggesting a critical role of AgRP in ghrelin-induced food consumption (Chen et al., 2004). The ghrelin receptor, the growth hormone secretagogue receptor 1 A (GHS-R1A) is highly expressed in many hypothalamic nuclei including the ARC (Zigman et al., 2006), and co-localizes in almost all AgRP/NPY neurons in this area (Willesen et al., 1999). GHS-R1A is also abundant in reward-linked brain regions such as the ventral tegmental area (VTA) (Abizaid et al., 2006, Zigman et al., 2006), and has been found to be co-localized with a sub-population of dopaminergic neurons in this area (Abizaid et al., 2006). Systemically administered ghrelin targets the VTA dopamine neurons, including those that project to the nucleus accumbens (NAc) and that confer reward. Thus, ghrelin has been shown to increase NAc dopamine release in mice (Jerlhag et al., 2006) and to increase the neuronal response in brain areas linked to reward in humans (Malik et al., 2008). Thus, ghrelin could influence reward-linked appetitive and consummatory behaviours by engaging this pathway. Consistent with this, ghrelin microinjected into the VTA increases food consumption and motivated behavior for food (Skibicka et al., 2011, Skibicka et al., 2013).
It seems clear that ghrelin may increase food intake through discrete neuronal circuits with divergent reinforcing properties. Some of these neuronal circuits may induce food consumption by motivated behaviors arisen from learning that consuming food will alleviate negative valence (e.g. AgRP/NPY neurons in the ARC), or by motivated behaviors linked to anticipation of positive valence and reward once food is consumed (e.g. through the mesolimbic dopamine pathway). Here, we sought to elucidate the overall reinforcing properties of ghrelin. Specifically, we explored whether ICV ghrelin is able to condition preference or avoidance in place and flavor preference paradigms.
2. Experimental procedures
2.1. Animals
Adult male Sprague-Dawley rats (Charles River Laboratories, Sulzfeld, Germany) were used. Body weight at the time of surgery was 220–310 g. Animals were kept under standardized non-barrier conditions on a 12/12 h light/dark cycle at 20–22 °C and 50% humidity. On arrival at the animal facility, animals had ad libitum access to standard maintenance chow (SDS RM1 diet, Special Diet Services, Witham, Essex, UK). Water was available ad libitum. The animal procedures were approved by the local ethics committee for animal care in Gothenburg, Sweden (Göteborgs djurförsöksetiska nämnd; permit number 45–2014) and were conducted in accordance with guidelines.
Adult male C57Bl/6 J mice (Envigo, Bicester, UK) were used, with body weight between 25–32 g at the time of surgery. Mice were maintained in standardized non-barrier conditions, on a 12/12 h light/dark cycle, at 22 °C ±1 °C and 45% ±10% humidity. Standard maintenance chow (SDS RM1 diet) and water were available ad libitum. Procedures on mice were carried out in accordance with the Animal (Scientific Procedures) Act 1986 (UK) and approved by the University of Manchester Animal Welfare and Ethics Review Board.
2.2. Guide cannula implantation
Rats were anaesthetized with a combination of Rompun® vet. 10 mg/kg (Bayer, Leverkusen, Germany) and Ketaminol® vet. 75 mg/kg (Intervet, Boxmeer, Netherlands) and placed in a stereotaxic frame. The skull bone was exposed and the skull sutures were identified. Holes for guide cannulae and anchoring screws were drilled through the skull. A 26-gauge cannula was positioned according to stereotactic coordinates and fixed in place with anchoring screws and dental cement (Dentalon, Heraeus Kulzer, Hanau, Germany). The guide cannula was placed 2 mm dorsal to the lateral ventricle and the following coordinates were used: 0.9 mm posterior to bregma, ± 1.6 mm lateral to the midline and 2.5 mm ventral of the skull surface. At injection, the injector used was 2 mm longer than the guide cannula to target the lateral ventricle. The injection volume was always 2 µl. After surgery the rats received an analgesic (Rimadyl® vet. 5 mg/kg, Orion Pharma Animal Health, Sollentuna, Sweden) and were singly housed during >4 day recovery period. To validate cannula placement in the lateral ventricle, conscious rats were injected with 20 ng angiotensin II (1158, Tocris, Bristol, U.K) for which correct placement is indicated by a dipsogenic (immediate water drinking) response (Epstein et al., 1970).
Mice were anaesthetized with 3% isoflurane (Abbot Abbvie Ltd, Maidenhead, UK) in oxygen (1500 ml/min) and placed in a stereotaxic frame. Throughout surgery, mice were maintained at the correct depth of anaesthesia using between 1–2% isoflurane in oxygen (800 ml/min). The skull bone was exposed and the skull sutures identified. A small screw was inserted into the left parietal plate of the skull. A hole was drilled 0.4 mm posterior to bregma, 1 mm lateral to the midline, through which a sterile guide cannula was implanted at a depth of 1.2 mm ventral to the skull surface (made in house from a 23-gauge needle cut to length). The guide cannula was fixed in place with the anchoring screw and dental cement (Simplex Rapid Powder, Kemdent, Swindon, UK / methyl methacrylate, Metrodent, Huddersfield, UK). At injection, the injector used was 0.5 mm longer than the guide cannula, to target the lateral ventricle. Injection volume was 2 µl.
2.3. Conditioned place preference/avoidance (CPP/CPA)
CPP/CPA testing in rats was performed using an apparatus composed of two chambers with distinct visual and tactile qualities and separated by a guillotine door (Med Associates Inc, Fairfax, VT, USA). One chamber was white and had a smooth-surface plastic floor while the other was black and had a rugged-surface plastic floor. Time spent in each chamber was recorded using infrared beams. In the morning of day 1, rats performed a pretest where they had access to both chambers and could freely explore both chambers for 20 min and initial chamber preference was recorded. A semi-unbiased design was used, in which ghrelin injection was paired with the most preferred chamber in half the rats and paired with the least preferred chamber in the other half. This design was used because we did not know at outset whether ghrelin would induce a CPP, a CPA or neither. On days 2–5, conditioning sessions were performed with one session in the morning and one in the afternoon (8 sessions in total), during which the rats only had access to one chamber for 20 min. Ghrelin (2 µg in 2 µl; 1465, Tocris, Bristol, U.K) was injected ICV in the morning and vehicle (aCSF; Tocris, Bristol, U.K) was injected ICV in the afternoon or vice versa. After injection, rats were immediately placed in the assigned chamber. In the morning of day 6, a test session was performed in which the rats again had free access to both chambers for 20 min. Time spent in each chamber was recorded. The rats always had free access to normal chow between sessions. Drinking water was always available, both during and between sessions. Two identical CPP/CPA experiments were performed with different sets of rats, with the exception that food was either absent (n=23) or present (n=11) during all sessions. In addition, a biased design was used in the second experiment in which ghrelin was injected in the most preferred chamber. The design was switched from semi-unbiased to biased since we then had information about ghrelin׳s reinforcing properties.
Conditioned place preference in mice was performed using an apparatus composed of two chambers with distinct visual and tactile qualities, connected by a brightly lit corridor (Harvard Biosciences/Biochrom Ltd., Cambridge, U.K). The lighter chamber consisted of a smooth grey floor and grey striped walls, whilst the darker chamber consisted of a rough black floor and black spotted walls. Time spent in each chamber was monitored by video cameras mounted directly above the apparatus, connected to a computer using tracking software (Smart v3.0, Panlab, Biochrom Ltd.). On the morning of day 1, mice (n=15) were given access to the full apparatus and could freely explore both chambers for 20 min, and initial pretest side preference was recorded. A biased design was used in mouse studies, whereby ghrelin was injected in the most preferred chamber, in order to complement the second rat study design. On day 2–5, conditioning sessions were performed, one in the morning and one in the afternoon (8 sessions in total), where mice were restricted to one chamber for 20 min. Ghrelin (1 µg in 2 µl; 1465, Tocris) was injected ICV in the morning and vehicle (saline, Braun, Melsungen, Germany) was injected ICV in the afternoon, or vice versa on alternate days. After injection, mice were immediately placed in the assigned chamber. On the morning of day 6, a test session was performed where the mice were again given free access to the full apparatus for 20 min, and time in each chamber monitored as before. Mice had ad libitum access to normal chow between sessions, when intake was measured.
2.4. Conditioned flavor preference/avoidance (CFP/CFA)
The protocol adopted was closely aligned to that used by Betley et al. to explore CFP/CFA in mice receiving optogenetic stimulation of AgRP neurons (Betley et al., 2015). Rats (n=20) were offered a free choice of two differently flavored (orange and strawberry) low-calorie (0.04 kcal/g), gelatin-based dessert gels (Jell-O Sugar Free, Kraft Heinz Foods Company, Chicago, IL, USA) during 1 h in the morning and 1 h in the afternoon. The positions of the two gels were switched after 30 min. Consumption of the two gels was measured and the initial flavor preference of each rat was determined by calculating the average preference of both sessions (9 rats preferred orange flavor and 11 rats preferred strawberry flavor). The most preferred flavor was subsequently paired with ghrelin injection and the least preferred flavor was paired with vehicle injection. This biased design was chosen because of information from CPPA/CPA experiments in which an avoidance was expected. Conditioning was performed twice a day for 4 days, one session in the morning and one session in the afternoon. Ghrelin (2 µg in 2 µl) was injected ICV in the morning and vehicle (aCSF) was injected ICV in the afternoon or vice versa. After injection, rats were immediately introduced to one of the flavored gels in a limited amount and had access to the gel for 30 min. The total amount of gel consumed over the 8 conditioning sessions did not significantly differ between flavors (data not shown). After conditioning, again the rats were offered a free choice of the two differently flavored gels for 1 h in the morning, and the amount of each gel was measured and preference was calculated. The positions of the two gels were switched after 30 min. The rats always had free access to normal chow between sessions. Drinking water was always available, both during and between sessions.
2.5. Pica response malaise test
The ingestion of kaolin, which has no nutritional value, reflects a state of visceral illness or nausea in rats and other animals incapable of vomiting (Mitchell et al., 1976, Takeda et al., 1993). In the pica test, the rats (n=22) were offered non-nutritive kaolin pellets (Research Diets Inc, New Brunswick, USA) in addition to normal chow for 3 days. On the following morning rats received either an ICV ghrelin injection (n=11) or an ICV vehicle injection (n=11) and the consumption of both normal chow and kaolin was measured at 3 h, 6 h and 24 h after injection.
2.6. Statistical analysis
Data were analysed using a paired Student׳s t-test in all CPP/CPA and CFP/CFA studies. An unpaired Student׳s t-test was used in the pica response study. Values are mean ± s.e.m. P < 0.05 was considered significant.
3. Results
3.1. ICV ghrelin injection conditions a place avoidance in rats and mice
To investigate if ghrelin administered ICV acts as a positive or negative reinforcer, we performed a semi- unbiased place preference test in which ICV ghrelin injection was conditioned to the most preferred chamber in half of the rats and to the least preferred chamber in the other half using a two-chamber CPP/CPA apparatus. In a pre-test, rats (n=23) spent 605±43 s out of a total of 1200 s (20 min) in the chamber that was subsequently paired with ghrelin but, after conditioning, rats spent only 111±16 s in that chamber, a decrease of 82% (P<0.001) (Figure 1A).
Figure 1.

ICV ghrelin conditions place avoidance in rats. Conditioned place avoidance (in seconds) for a chamber conditioned to ICV ghrelin in a pre-test session performed before conditioning and in a test session performed after conditioning. Food was absent (A) or present (B) during conditioning, pre-test and test. (C) When food was present, ghrelin-injected rats ate more during the conditioning sessions compared with vehicle-injected rats. In A: n=23 and in B,C: n=11.*** P<0.001.
To investigate if the negative experience of ICV ghrelin in rats could be alleviated by the consumption of food, we performed a similar experiment with the exception that food was present during conditioning. We used a biased design (as we now knew that ghrelin induced a place avoidance when rats did not have access to food during conditioning) and ghrelin injection was paired with the most preferred chamber. Time spent in the chamber that had been paired with ghrelin injection was also decreased dramatically (by 77%; P<0.001) (Figure 1B) compared with pre-test values, even though ghrelin-treated rats had an 12-fold (P<0.001) increased accumulated food intake (2.6 ± 0.4 g) during the conditioning sessions, compared with vehicle treatment (0.2±0.1 g; Figure 1C). Rats (n=11) spent 197±82 s in the ghrelin-paired chamber compared with 860 ± 53 s during the pre-test (Figure 1B).
Given that previous studies reported effects of peripheral ghrelin injection to induce a CPP or CPA in mice, we decided also to perform CPP/CPA testing in mice. In a similar experiment using mice, we saw a similar effect of ICV ghrelin to condition a place aversion, using a biased design with ghrelin conditioned to the most preferred side in all mice. In a pre-test, mice (n=15) spent 647±17 s out of a total of 1200 s (20 min) in the chamber that was subsequently paired with ghrelin, but after conditioning mice spent only 518±28 s in that chamber, a decrease of 20% (P<0.001) (Figure 2). In these experiments the mice had no access to food during the conditioning session, but did have access between sessions when in their home cage. Between conditioning sessions, ghrelin-treated mice ate 0.71±0.06 g chow, compared with saline-injected mice that ate 0.08±0.03 g (P<0.001), demonstrating the expected orexigenic effect of the hormone.
Figure 2.

ICV ghrelin conditions a place avoidance in mice. Conditioned place avoidance (in seconds) for a chamber conditioned to ICV ghrelin in a pre-test session, performed before conditioning and in a test session performed after conditioning (n=15). *** P<0.001.
3.2. ICV ghrelin administration conditions a flavor avoidance in rats
We sought to test whether central ghrelin could influence the preference for gustatory cues. To this end we performed a biased conditioned flavor preference test in ad libitum-fed rats (n=20) in which ICV ghrelin injection was conditioned to a non-nutritive gel with a distinct flavor. After conditioning, the preference for the flavor that had been conditioned to ICV ghrelin injection (31±7%) was dramatically reduced by over half (P<0.001) compared with the initial preference (71±3%), which was determined prior to conditioning (Figure 3). The avoidance of the flavor associated with ghrelin injection indicates, once again, that ghrelin acts as a negative reinforcer, carrying a negative-valence signal.
Figure 3.
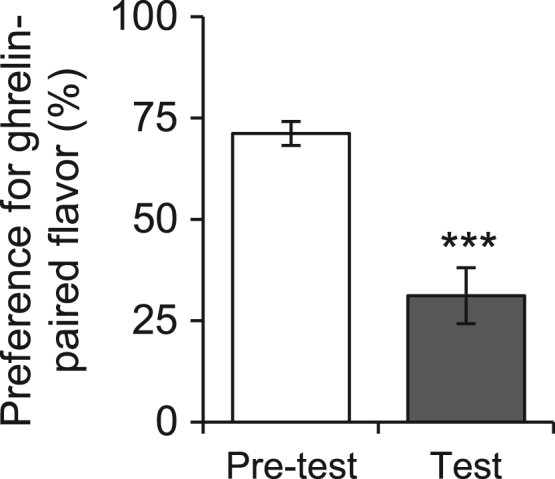
ICV ghrelin conditions flavor avoidance. Conditioned flavor preference for ghrelin-conditioned flavor in a pre-test session performed before conditioning and in a test session performed after conditioning (n=20). *** P<0.001.
3.3. ICV ghrelin does not appear to cause malaise in the pica test in rats
To rule out the possibility that ICV ghrelin injection could evoke malaise or nausea, we performed a pica response test where non-nutritive kaolin intake was measured in addition to normal chow after central ghrelin injection to rats. Centrally injected ghrelin did not induce consumption of kaolin at 3 h, 6 h or 24 h after injection (Figure 4B), while the same ghrelin dose increased normal chow consumption at 3 h and 6 h after injection by 81% (P<0.001) and 75% (P<0.001), respectively, compared with vehicle injection (Figure 4A). This experiment indicates that the central effects of ghrelin to induce negative valence reported above is independent from strong feelings of malaise or nausea.
Figure 4.

Pica test: ICV ghrelin does not induce malaise in rats. ICV ghrelin (n=11 rats), injected at the same dose as used in the conditioned place/flavor preference experiment (2 µg) (A) increased food consumption at 3 h and 6 h post injection compared with vehicle controls and (B) did not simultaneously increase kaolin consumption. *** P<0.001.
4. Discussion
Here we demonstrate that, in both rats and mice, ICV ghrelin acts as a negative reinforcer, consistent with its role as a circulating hunger hormone. This is evidenced by the fact that the animals avoid both a chamber previously paired to ghrelin injection (CPP/CPA testing) and also a flavor paired to ghrelin injection (CFP/CFA testing). We interpret these findings to suggest that ghrelin may promote food intake by engaging pathways involved in negative reinforcement.
Our findings resonate with studies showing that AgRP/NPY neurons (an orexigenic cell group that are a known target for ghrelin) transmit a negative valence teaching signal (Betley et al., 2015). Using optogentic approaches, it has been shown that mice dislike having their AgRP/NPY neurons activated and eat because this suppresses the activity of the neurons (Betley et al., 2015). Here we show that ghrelin, like AgRP/NPY neuron activation, could also operate as a negative-valence teaching signal, although further studies would be necessary to confirm that these effects of ghrelin are routed through the AgRP signaling system (Figure 5).
Figure 5.
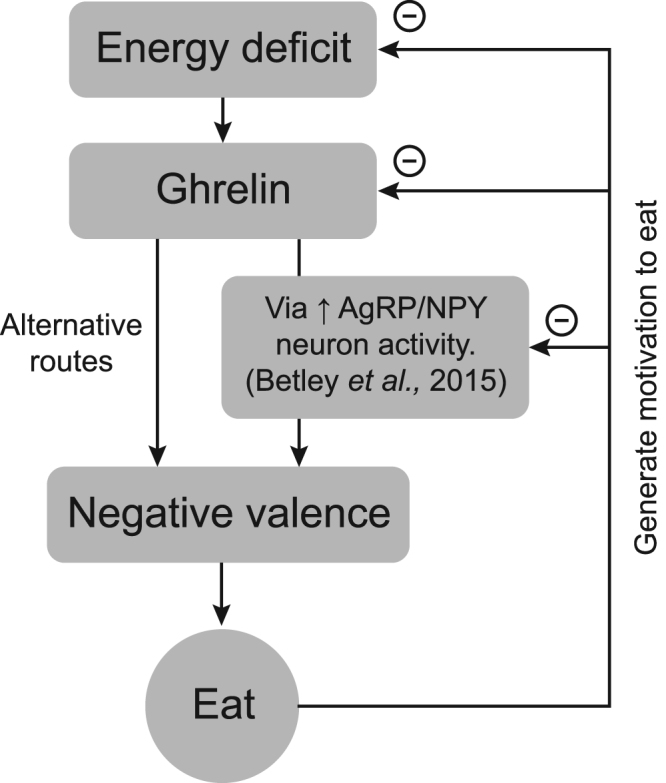
Ghrelin may act as a negative reinforcer to promote food consumption. In a state of energy deficit, ghrelin levels are elevated. Ghrelin promotes food consumption, possibly by inducing a negative-valence state through the activation of AgRP/NPY neurons in the arcuate nucleus, or through alternative routes. A ghrelin-induced initiation of food consumption may be generated by the learned alleviation of negative valence that occurs once the food has been eaten. This negative feedback, acting on components (e.g. ghrelin, NPY/AgRP neurons) that produce the negative-valence signal, may motivate food consumption.
To investigate if the negative reinforcing properties of ghrelin are lost when food is available, we performed a CPP/CPA experiment in rats when ICV ghrelin conditioning occurred in the presence of food. We found that the negative reinforcing properties of ghrelin were independent of the availability of food during the conditioning and test sessions. This is surprising given that the activity of AgRP/NPY neurons rapidly decreases just as food is recognized, and that the reduced neuron activity is maintained during the consumption of food (Betley et al., 2015, Chen et al., 2015). In theory, if the negative reinforcing properties of ghrelin are transduced through AgRP/NPY neurons, having food available during conditioning would reduce these. It may be that ghrelin engages alternative pathways to induce negative emotions by bypassing these neurons. Alternatively, it could be that having food available, has not yet reduced AgRP/NPY neuron activity to a level that makes it possible to over-ride ghrelin׳s effects to induce a CPA, in this particular paradigm. Indeed, Betley et al. show that AgRP/NPY neuron activity is still elevated 20 min after ghrelin injection in ad libitum-fed mice (Betley et al., 2015). Nevertheless, this question need to be further investigated.
We identified two previous studies that investigated the reinforcing properties of ghrelin in CPP/CPA paradigms, both in mice. In one study, systemic administration of ghrelin induced a CPP (Jerlhag, 2008) and, in the other, it induced a CPA (Lockie et al., 2015). However, in the latter study, when chow pellets were available during conditioning and test sessions, ghrelin induced a CPP (Lockie et al., 2015). The discrepancies and similarities between these and our current study could be explained by the route of administration (systemic vs. central) or the doses used resulting in differential capacity of ghrelin to activate AgRP/NPY neurons in the ARC. Systemic administration of a higher dose of ghrelin induces a CPA (Lockie et al., 2015), while a lower dose induced a CPP (Jerlhag, 2008). Similarly, systemically administered ghrelin, in the presence of food, induced a CPP (Lockie et al., 2015), while central administration, in the presence of food, as shown here, induces a CPA. Again, centrally administered ghrelin may have a differential capacity to activate AgRP/NPY neurons than the systemically administered ghrelin. Indeed, the passage of ghrelin through the blood-brain barrier has been shown to be complex (Banks et al., 2002).
An alternative explanation could involve divergent dopamine release in the NAc as a result of systemic or centrally administered ghrelin. Indeed, ghrelin administration, both central and peripheral, has been shown to induce dopamine release in the NAc even though the magnitude of the release is problematic to compare between studies (Abizaid et al., 2006, Jerlhag, 2008). Although clear that peripheral ghrelin can access the CNS (Schaeffer et al., 2013) we do not yet know the extent to which peripheral versus central ghrelin can access the VTA and act on dopaminergic neurons.
Given that the literature is not in agreement on whether ghrelin conditions preference or aversion, we investigated this further using a different conditioning paradigm, namely conditioned flavor preference/avoidance (CFP/CFA). In line with our results for the CPP/CPA test, ghrelin induces a CFA in a non-nutritive gel-based paradigm. This demonstrates that the cues associated with the negative reinforcing properties of ICV ghrelin also involve olfactory and gustatory cues in addition to visual and tactile cues shown in the CPP paradigm. In line with our observation, channel rhodopsin-mediated photo activation of AgRP/NPY neurons in the ARC produced flavor avoidance in a similar non-nutritive, gel-based paradigm (Betley et al., 2015). In addition, central administration of NPY has been shown to induce a conditioned flavor aversion at doses that stimulate food intake (Sipols et al., 1992), which further supports our findings.
Our data, indicating that rodents may find it unpleasant to receive an ICV injection of ghrelin raised concerns that the animals may be experiencing malaise. This we explored using the pica test and found that the ingestion of kaolin clay pellets was unchanged in rats receiving central ghrelin at a dose that simultaneously increases chow pellet consumption (the same used here in CPP/CFP experiments). Kaolin ingestion, or the pica response, is an indicator of nausea in rodents as they do not possess the ability to vomit (Mitchell et al., 1976, Takeda et al., 1993). Thus, the evoked avoidance by ghrelin in the CPP and CFP experiments is likely not due to any induced nausea caused by centrally administered ghrelin.
In summary, we present evidence that centrally administered ghrelin to the brain ventricular system conditioned avoidance using both visual and tactile cues as well as olfactory and gustatory cues, without inducing nausea. This indicates that ghrelin may drive the motivation to seek and consume food through negative reinforcement.
Role of funding source
None of the funding sources had any role in the study design, data collection, data analysis, writing of the manuscript or the decision to submit the manuscript for publication.
Contributors
The project idea was initiated by SLD, involving a collaboration with SML. ES and SLD planned the rat experiments that were undertaken by ES and ML. SL planned the mouse experiments that were undertaken by CC. ES wrote the first draft of the manuscript and all authors contributed to the final text.
Conflict of interest
All authors declare to have no conflict of interest.
Acknowledgements
SLD supported by EC Framework 7 grant Nudge-it (607310), the Swedish Research Council for Medicine (Vetenskapsrådet, grant number 2016-02195), and by Avtal om Läkarutbildning och Forskning (ALFBGB-138741). SML and CC are supported by BBSRC project (BB/N007549/1) and postgraduate studentship funding (BBSRC DTP), respectively.
References
- Abizaid A., Liu Z.W., Andrews Z.B., Shanabrough M., Borok E., Elsworth J.D., Roth R.H., Sleeman M.W., Picciotto M.R., Tschop M.H., Gao X.B., Horvath T.L. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D., Betley J.N., Su H.H., Sternson S.M. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Tschop M., Robinson S.M., Heiman M.L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Betley J.N., Xu S., Cao Z.F., Gong R., Magnus C.J., Yu Y., Sternson S.M. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.Y., Trumbauer M.E., Chen A.S., Weingarth D.T., Adams J.R., Frazier E.G., Shen Z., Marsh D.J., Feighner S.D., Guan X.M., Ye Z., Nargund R.P., Smith R.G., Van der Ploeg L.H., Howard A.D., MacNeil D.J., Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lin Y.C., Kuo T.W., Knight Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M.A., Smith R.G., Diano S., Tschop M., Pronchuk N., Grove K.L., Strasburger C.J., Bidlingmaier M., Esterman M., Heiman M.L., Garcia-Segura L.M., Nillni E.A., Mendez P., Low M.J., Sotonyi P., Friedman J.M., Liu H., Pinto S., Colmers W.F., Cone R.D., Horvath T.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Dickson S.L., Luckman S.M. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- Epstein A.N., Fitzsimons J.T., Rolls B.J. Drinking induced by injection of angiotensin into the rain of the rat. J. Physiol. 1970;210:457–474. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict. Biol. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E., Egecioglu E., Dickson S.L., Andersson M., Svensson L., Engel J.A. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict. Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S., Maratos-Flier E., Roth B.L., Lowell B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C.B., Snape A.C., Baudoin F.M., Luckman S.M. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lockie S.H., Dinan T., Lawrence A.J., Spencer S.J., Andrews Z.B. Diet-induced obesity causes ghrelin resistance in reward processing tasks. Psychoneuroendocrinology. 2015;62:114–120. doi: 10.1016/j.psyneuen.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Malik S., McGlone F., Bedrossian D., Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mitchell D., Wells C., Hoch N., Lind K., Woods S.C., Mitchell L.K. Poison induced pica in rats. Physiol. Behav. 1976;17:691–697. doi: 10.1016/0031-9384(76)90171-2. [DOI] [PubMed] [Google Scholar]
- Schaeffer M., Langlet F., Lafont C., Molino F., Hodson D.J., Roux T., Lamarque L., Verdie P., Bourrier E., Dehouck B., Baneres J.L., Martinez J., Mery P.F., Marie J., Trinquet E., Fehrentz J.A., Prevot V., Mollard P. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl. Acad. Sci. USA. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipols A.J., Brief D.J., Ginter K.L., Saghafi S., Woods S.C. Neuropeptide Y paradoxically increases food intake yet causes conditioned flavor aversions. Physiol. Behav. 1992;51:1257–1260. doi: 10.1016/0031-9384(92)90317-u. [DOI] [PubMed] [Google Scholar]
- Skibicka K.P., Hansson C., Alvarez-Crespo M., Friberg P.A., Dickson S.L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Skibicka K.P., Shirazi R.H., Rabasa-Papio C., Alvarez-Crespo M., Neuber C., Vogel H., Dickson S.L. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin׳s effect on food reward but not food intake. Neuropharmacology. 2013;73:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Sternson S.M., Eiselt A.K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 2016 doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- Takahashi K.A., Cone R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Takeda N., Hasegawa S., Morita M., Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol., Biochem., Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Willesen M.G., Kristensen P., Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Zigman J.M., Jones J.E., Lee C.E., Saper C.B., Elmquist J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]


