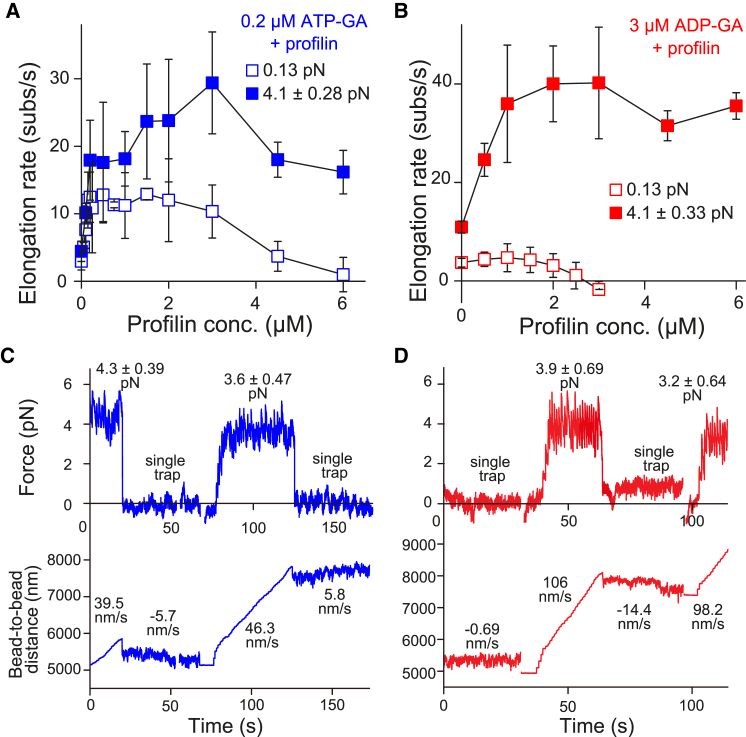
Figure 6.
Involvement of profilin in force-dependent elongation of actin filaments. (A and B) Elongation rates are shown at various profilin concentrations with 0.2 μM ATP-G-actin (A) or 3 μM ADP-G-actin (B). Open and solid symbols represent single- and double-trap experiments, respectively: n = 20, 6, 11, 6, 17, 6, 8, 3, 9, 3, 11, 15, 6, and 6 (single-trap) and 11, 6, 12, 3, 5, 4, 7, 12, 5, and 10 (double-trap) for ATP-G-actin; 5, 4, 5, 3, 4, 3, and 17 (single-trap) and 14, 5, 7, 5, 19, 4, and 3 (double-trap) for ADP-G-actin, from lower to higher profilin concentration. Error bars indicate mean ± SD. Tensile force is 4.1 ± 0.28 (ATP-G-actin) and 4.1 ± 0.33 pN (ADP-G-actin) (mean ± SD). (C and D) Shown here is the reversibility of force-dependent actin filament elongation. The elongation rate force dependence observed upon excess profilin concentration is reversible. The actin filaments, which elongated very slowly, did not elongate, or depolymerized in the single-trap experiments (almost no tension), were shifted into the fast-elongation state by switching to the double-trap experiment (strong tensile force). Upon the mDia1 bead release, actin filament elongation returned to a very slow rate. (C) Shown here are 0.2 μM ATP-G-actin and 6 μM profilin. (D) Shown here are 3 μM ADP-G-actin and 3 μM profilin. To see this figure in color, go online.