Abstract
Background
Recovery from post-stroke aphasia is a long and complex process with an uncertain outcome. Various interventions have been proposed to augment the recovery, including constraint-induced aphasia therapy (CIAT). CIAT has been applied to patients suffering from post-stroke aphasia in several unblinded studies to show mild-to-moderate linguistic gains. The aim of the present study was to evaluate the neuroimaging correlates of CIAT in patients with chronic aphasia related to left middle cerebral artery stroke.
Material/Methods
Out of 24 patients recruited in a pilot randomized blinded trial of CIAT, 19 patients received fMRI of language. Eleven of them received CIAT (trained) and eight served as a control group (untrained). Each patient participated in three fMRI sessions (before training, after training, and 3 months later) that included semantic decision and verb generation fMRI tasks, and a battery of language tests. Matching healthy control participants were also included (N=38; matching based on age, handedness, and sex).
Results
Language testing showed significantly improved performance on Boston Naming Test (BNT; p<0.001) in both stroke groups over time and fMRI showed differences in the distribution of the areas involved in language production between groups that were not present at baseline. Further, regression analysis with BNT indicated changes in brain regions correlated with behavioral performance (temporal gyrus, postcentral gyrus, precentral gyrus, thalamus, left middle and superior frontal gyri).
Conclusions
Overall, our results suggest the possibility of language-related cortical plasticity following stroke-induced aphasia with no specific effect from CIAT training.
MeSH Keywords: Aphasia, Language Disorders, Magnetic Resonance Imaging, Stroke, Treatment Outcome
Background
Aphasia is considered one of the most devastating deficits caused by stroke. Spontaneous recovery from a stroke-induced aphasia is a long-term process that involves extensive and complex functional reorganization of the cortical language network with variable participation from the dominant and non-dominant hemispheres [1]. The majority of improvements typically occur in the first weeks after stroke with about 50% of patients left with long-term deficits that significantly affect the ability to communicate or to participate in the activities of daily living [2]. While spontaneous recovery may be observed even years after stroke, substantial recovery more than a year after stroke is very unlikely and, thus, rehabilitative interventions are necessary to augment the declining rate of recovery. Although studies have demonstrated that therapies administered during the first year after stroke improve recovery [3,4], relatively little is known about the possibility of treatment-induced improvements in chronic post-stroke aphasia or their cortical correlates [5].
Historically, rehabilitation strategies for chronic post-stroke aphasia have included multi-modality compensatory strategies (e.g., gesturing, drawing, writing), and low- or high-technology augmentative and alternative communication (AAC) strategies, all of which are expected to slowly be abandoned by patients as the verbal language functions return. These interventions likely help patients in the short-term but are thought to inhibit long-term recovery via phenomenon of “learned non-use” [6]. In order to reverse learned non-use, an alternative therapy – constraint-induced aphasia therapy (CIAT) – was developed. CIAT (also called constraint-induced language therapy or CILT) is a language-based program [7–11] adapted from post-stroke motor constraint therapy [12]. In the CIAT model, it has been postulated that the behavior of attempting to speak without success leads to communication frustration and more dependence on compensatory techniques. This then results in less verbal communication and limited cortical stimulation in brain regions supporting language. Within the CIAT therapy environment, recovery is facilitated by positive reinforcement from group members, clinician cueing and shaping, and positive social interactions [7,9]. The supportive environment and frequent speaking opportunities encourage more verbal attempts and stimulate cortical reorganization [13].
Several studies have evaluated the neural correlates of CIAT [13–20]. Of those studies, only one [16] included a control group that was composed exclusively of healthy participants (i.e., with no history of stroke or any brain damage). Therefore, it is uncertain whether the observed changes were specifically related to the intervention or whether they were the results of the intense training and social interaction both of which are integral parts of CIAT. Thus, the aim of the present study was to examine the benefits of intensive language training in CIAT on the cortical correlates of post-stroke recovery in patients with chronic (> one year) post-stroke aphasia who participated in a randomized and blinded trial of CIAT and who were able to receive fMRI [7]. We accomplished this by comparing the fMRI language activation patterns of participants who received CIAT to the control stroke participants with aphasia who had not received any intervention during that period of time but who were scanned and received language testing at the same time points as the CIAT participants. The working hypothesis, grounded in previous research, was that improvement in linguistic and communicative abilities in the CIAT group would be associated with increased lateralization of the fMRI language activations to the affected left hemisphere; in contrast, lack of improvement in linguistic abilities in the control (observation) group would be associated with stable, right > left language lateralization.
Material and Methods
Participants
All patients admitted to acute care hospitals participating in the study (The University of Cincinnati Hospital in Cincinnati, OH, St. Elizabeth Medical Center in Erlanger, KY, and the University of Alabama at Birmingham Hospital in Birmingham, AL) and who presented with aphasia were considered for participation. Admission logs for stroke diagnoses were screened daily. Prior to enrollment, the diagnosis of single ischemic stroke in the left middle cerebral artery (LMCA) distribution was confirmed by medical record review including admission notes for the incident stroke and the report of the imaging of the brain (CT or MRI) that was obtained as part of the admission. If deemed a potential candidate, after obtaining verbal consent from the treating physician, a participant and/or their caregiver(s) were approached for potential participation. If interested, a screening token test (TT) was administered to determine eligibility and grossly estimate aphasia severity: TT=40–37 was categorized as mild, TT=36–17 was categorized as moderate, and TT=16–0 was categorized as severe [21]. TT was used for screening purposes only and detailed linguistic testing was performed once eligibility was determined. All patients with at least mild aphasia were offered participation. The exclusion criteria were history of degenerative (e.g., dementia or Parkinson disease) or metabolic disorder (e.g., encephalopathy) or supervening medical illness (e.g., brain tumor or other cancer), history of severe depression or other mental illness, and positive pregnancy test in women of childbearing age. The institutional review boards of all participating institutions approved these procedures.
Thirty-two individuals were identified as potential candidates and were interviewed. Five potential participants were excluded after interviews due to the presence of exclusion criteria (see our recent study for the CONSORT statement [7]). The format and the goals of the CIAT program were explained to all participants at the time of obtaining the informed consent. All patients indicated their understanding of the goals of the program prior to signing the informed consent; they also understood that they may be randomized to a no-intervention group, and that the follow-up testing would need to be performed. Three more participants were excluded after completion of TT screening. Of the 24 remaining participants, 19 were eligible to receive MRI scanning and successfully completed three scanning sessions – one within a week before the start of the intervention, one within a week after completing the intervention, and one at three months after the intervention; scanning in the observation group was performed at the same time points relative to enrollment). Eleven of these participants (mean age=58, SD=10.63, 5 females) were randomized to receive CIAT (trained group) and the remaining eight (mean age=50.3, SD=13.34, 3 females) were randomized to the control group (untrained group). The groups did not significantly differ in age (p=0.18) or in TT results (p=0.59). Participants’ handedness index was determined using the Edinburgh Handedness Inventory [22]. Prior to the stroke, 17 participants were right-handed (handedness index > 91), two participants (one from the control group and one from the CIAT group) were considered to have atypical handedness (handedness index were −100 and 50, respectively [22,23]. Participants were asked not to take part in any other intervention during their involvement in the study and all complied. Demographic and clinical data of the participants are provided in Table 1.
Table 1.
Demographic characteristics of study participants.
| Control group (n=8) | CIAT group (n=11) | Healthy group (n=38) | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) – mean (SD) | 50 | −13.3 | 58 | −10.6 | 54 | −10.85 | 0.183 |
| White – n (%) | 7 | −87.5 | 10 | −90 | 37 | −97.3 | 0.812 |
| Non-Hispanic – n (%) | 8 | −100 | 11 | −100 | 38 | −100 | – |
| Male – n (%) | 5 | −62.5 | 6 | −54.5 | 22 | −57.9 | 0.728 |
| Past medical history – n (%) | |||||||
| History of HTN | 2 | −25 | 4 | −36.6 | 0 | 0 | 0.552 |
| History of DM | 1 | −12.5 | 1 | −9 | 0 | 0 | 0.812 |
| History of high cholesterol | 4 | −50 | 6 | −54.5 | 0 | 0 | 0.845 |
| History of CAD | 0 | 0 | 2 | −18.8 | 0 | 0 | 0.125 |
| History of MI | 1 | −12.5 | 1 | −9 | 0 | 0 | 0.812 |
| Smoking | 4 | −50 | 5 | −0.45 | 0.845 | ||
| Alcohol abuse | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Drug abuse | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Prior stroke | 2 | −25 | 0 | 0 | 0 | 0 | 0.051 |
| Severity – n (%) | |||||||
| Mild aphasia | 2 | −25 | 4 | −36.6 | 0 | 0 | 0.596 |
| Moderate aphasia | 3 | −37.5 | 2 | −18.8 | 0 | 0 | 0.347 |
| Severe aphasia | 3 | −37.5 | 5 | −45.4 | 0 | 0 | 0.728 |
| Time since stroke (months) – median (IQR) | 41.9 | −30 | 60.2 | −48.9 | N/A | N/A | 0.33 |
HTN – hypertension; DM – diabetes mellitus; CAD – coronary artery disease; MI – myocardial infarction.
In addition to people with stroke, 38 healthy participants were recruited so that each stroke patient was matched to two healthy controls by age, gender, and handedness. Demographic data for healthy participants are also provided in Table 1.
CIAT training
CIAT is an intensive form of language-action therapy performed in a small group setting. This training includes 10 daily sessions, each four hours long, of tailored intervention designed to promote spoken language and to limit compensatory non-spoken strategies [7–9]. Sessions are supervised concurrently by at least two trained speech language pathologists. In a therapeutic game context, participants request picture cards from each other, by using descriptions of the depicted objects, and understand requests made by others and the therapist. Clues are provided to participants with increasing hierarchy (for details, see [7]).
Experimental design
Each CIAT participant was assessed three times: less than one week prior to training, within one week of competing training, and three months after training; observational group participants were scanned in parallel. Each session included data collected from two fMRI tasks: semantic decision/tone decision (SDTD; [24]) and covert verb generation (VG; [25]); both are fully detailed in the following text. Behavioral testing was also administered during each session and included the following tests: 1) the Peabody Picture Vocabulary Test, Fourth Edition (PPVT) to test receptive vocabulary, where participants were asked to select one out of a set of four pictures they thought best represented the meaning of the word orally presented by the examiner [26]; 2) the Boston Naming Test, Second Edition (BNT) to test word-finding and semantic retrieval processes, where participants were asked to name the picture presented by the examiner [27]. The PPVT and BNT were each summarized as a standardized score based on correct responses. We also obtained raw scores from 3) the Controlled Oral Word Association Test (COWAT) to test oral fluency, where participants were scored based on the number of words beginning with a given letter they could generate in one minute [28]; and 4) the Semantic Fluency Test (SFT) to test verbal fluency and semantic retrieval, where performance was scored based on the number of words the participant could generate in one minute for a given category [28,29]. To decrease the possibility of learning effects, different versions of the tests were rotated between the sequential examinations in stroke patients and were randomized to receive various versions of the tests to assure balanced distribution of all tests/versions used (except for BNT). In addition, the Mini-Communicative Activities Log (Mini-CAL), which is a subjective measure of communicative abilities [8,9] was administered twice, at session one and session three.
Functional MRI block-design tasks
The SDTD task is presented in 30-second blocks with two alternating conditions: the control condition (tone decision) and the active condition (semantic decision) [24,30,31]. The cycle of 30-second blocks was repeated five times (plus an initial 30-second control block used for MR signal equilibration that was not analyzed) [30,31]. The total duration of the entire task was five minutes and 30 seconds. In the tone condition, participants heard brief sequences of four to seven 500-Hz and 750-Hz tones every 3.75 seconds and responded with the non-dominant hand button pressed to any sequence containing either two 750-Hz tones (index finger) or other than two 750-Hz tones (middle finger). Similarly, in the active condition, participants heard spoken English nouns designating animals every 3.75 seconds and responded with a non-dominant hand button press (index finger) to stimuli that met two criteria: “native to the United States” and “commonly used by humans.” In all other cases, participants responded by pressing the middle finger button.
The verb generation task developed for this study was based on a task introduced by Petersen et al. [32]. As in our previous studies [33–35], the VG task consisted of alternating 30-second long blocks of active and control condition. During each active block, the participants were presented with a noun every six seconds (five nouns/test block) and were instructed to silently generate verbs associated with each noun (covert generation). During each control block, the participants were presented with a target tone every six seconds (five tones/control block) and were instructed to perform bilateral sequential finger tapping in response to each tone. The control blocks were designed to distract the participant and interrupt the process of covert verb generation between active blocks and to control for the auditory presentation of the noun during the test condition. The task began with a control condition, followed by alternating test and control conditions (five blocks of the active condition and six blocks of the control condition, starting with the control condition; the initial 30-second block was used for MR signal equilibration and not analyzed). Understanding of and ability to perform each task was confirmed by study staff before the participants entered the scanner.
Each participant performed two repetitions of each of the tasks. For the SDTD task, responses during the fMRI session were recorded for further analyses. For the VG task, a post-scan memory test was performed which consisted of 30 forced-choice questions in which the participant was instructed to identify those nouns that were presented during the active blocks. Rather than the performance of each participant, the goal for each of the fMRI tasks was to engage a maximum of brain language areas that remain functional after the stroke event.
MRI data acquisition
MRI data were acquired at the Cincinnati Children’s Hospital Medical Center on a 3.0 Tesla research-dedicated Philips MRI system located in the Imaging Research Center. The scanner was equipped with an audio-visual system for presentation of task stimuli (Avotec Systems Inc.). Echo planar imaging (EPI) fMRI scans were performed using thirty-two 4 mm thick axial slices covering the entire brain. EPI images were obtained using a T2*-weighted gradient-echo EPI pulse sequence (TR/TE=2000/38 ms, FOV=24.0×24.0 cm, matrix=64×64, slice thickness=4 mm). In addition, a high-resolution T1-weighted three-dimensional anatomical scan was obtained (TR/TE=8.1/3.7 ms, FOV=25.0×21.1×18.0 cm, matrix=252×211, flip angle 8°, slice thickness=1 mm) for localization of brain regions. For each fMRI run, 165 whole brain scans were acquired.
At the University of Alabama at Birmingham, MRI data were acquired on a research dedicated 3.0 Tesla Siemens MR System located in the Civitan Functional Neuroimaging Laboratory (CFNL) using a circular polarized head coil. EPI fMRI scans were performed using 30, 4 mm thick axial slices covering the entire brain. EPI images were obtained using a T2*-weighted gradient-echo EPI pulse sequence (TR/TE=2000/38 ms, FOV=24.0×24.0 cm, matrix=64×64, slice thickness=4 mm). In addition, a high-resolution T1-weighted three-dimensional anatomical scan was obtained (TR/TE=2300/2.17 ms, FOV=25.6×25.6×19.2 cm, matrix=256×256, flip angle 9°, slice thickness=1 mm) for localization of brain regions. For each fMRI run, 165 whole brain scans were acquired.
MRI data preprocessing
All imaging data were preprocessed and modeled using Matlab toolbox SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). For each stroke patient and each task (SDTD and VGT), fMRI data preprocessing was conducted according to the following steps:
For each run, the first 15 volumes (i.e., 30-second control block) were discarded.
Spatial discrepancy between volumes due to head motion was corrected using SPM12 realign. All runs from all three sessions were fed into to the realign algorithm as it contains an implicit co-registration step designed to correct spatial discrepancies between runs.
Within each run, temporal discrepancies between slices were corrected using the slice-timing function in SPM12.
Each anatomical scan was realigned onto its respective mean functional scan computed at step 2 using the coregister function in SPM12; anatomical scan obtained at session one was realigned to the functional scan obtained at session one with the same procedure performed for all scanning sessions.
Longitudinal toolbox [36] was used on all anatomical scans (one for each longitudinal time point). This toolbox computes an average anatomical scan that is corrected for the intensity and inhomogeneity artifacts usually seen in MRI data; deformation field maps corresponding to the deformation differences between the average anatomical and each one of the anatomical scans were created.
Deformation field maps created in step 5 were used to warp respective functional runs; e.g., deformation field map of the difference between the average anatomical and the anatomical scan obtained at session one were applied to the functional scan obtained at session one; the same procedure was applied to the sequential sessions.
Unified segmentation [37] computed on average anatomical scan generated in step 5 was used to normalize all three functional runs.
Finally, functional scans were spatially smoothed with an 8-mm full width half-maximum kernel.
Functional MRI data processing for healthy participants followed the same steps except for steps 5 and 6.
Functional MRI statistical analysis
For each participant and each task, a general linear model (GLM) analysis was performed by modeling the fMRI time-series from the block-design tasks as boxcar regressors convolved with the canonical hemodynamic response function (HRF). In addition, 24 movement regressors were included according to the Friston 24-parameter model [38] plus two regressors accounting for run number. Group random effects were computed using one-sample t-tests with SPM12. Group comparisons were carried out using two-sample t-tests in SPM12. In order to avoid potential issues due to participants’ brain lesions, lesion-frequency maps (see section Lesion-frequency maps and Figure 1) were used as masks and excluded from group comparison statistical analyses.
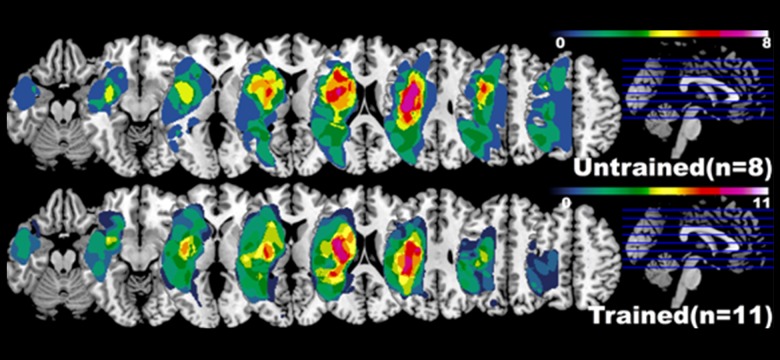
Figure1.
Lesion maps for both groups of stroke participants (trained and untrained). Each voxel value is the number of participants whose stroke lesion extends to that particular voxel (all pictures in neurological convention). See Methods section for detail.
Lateralization Index (LI)
It is now well recognized that language functions are lateralized within the hemispheres [39] and that stroke has a direct impact on language lateralization [1,34]. Lateralization index (LI) is commonly used to describe the hemispheric or regional distribution of activations in functional neuroimaging studies in response to specific fMRI task (e.g., language or memory) [34,40]. This index includes values between −1 and +1, with +1 being a purely left and −1 a purely right hemispheric activation. LIs were computed using LI-toolbox [41] on contrast maps obtained by combining HRF and derivatives contrasts. Parameters recommended by the authors were used for LI analyses. For each map, a threshold was computed using a bootstrap algorithm [42]. Values above that internal threshold are added in order to generate a global value for each hemisphere within a region of interest (mask). Then, the LI value was calculated based on the following equation (activation designates the number of voxels above the threshold):
For each participant and each contrast map (SDTD and VG), LI was calculated using six different masks: frontal, temporo-parietal, cerebellar, Broca area (atlas-based masks provided are with the LI-toolbox, see [41] for details), and two functional based on the results of the GLM analyses of the same fMRI tasks on healthy controls. Threshold control GLM contrast results (FWE p<0.05) were binarized in order to create the functional GLM masks (one mask for the SDTD task and one for the VG task). In order to obtain symmetrical GLM masks, binary values of the left hemisphere were mirrored over the right hemisphere and vice versa. For all masks, voxels within a 20 mm area around the midline (10 mm left and 10 mm right) in an axial plane were nullified (not included into the mask).
Behavioral data analysis
Extensive statistical analysis of behavioral data was previously performed by our group [7]. However, the number of participants included in the current manuscript was smaller due to MRI scanning constraints (24 individuals in the original publication versus 19 here (11 participating in CIAT and eight controls). Therefore, new statistical analyses were computed on behavioral data, using statistical functions implemented in Matlab 2014b (The Mathworks, Inc., Natick, MA, USA.) using only data from the included participants.
Lesion-frequency maps
We developed a Matlab script to compute the stroke-induced lesion area for each stroke patient [43]. Probabilistic tissue segmentation and image algebra (with naïve Bayes classification) were used to create feature maps encoding information about missing and abnormal tissue. All maps were binarized then summed into one image (separately for each group) in which the value of each voxel represents the frequency of lesion at this particular cortical location (Figure 1).
Results
Behavioral results
Repeated-measures ANOVAs were computed for each score with session (two sessions for mini-CAL and three sessions for all other scores) and group (trained versus untrained) as factors. Results are summarized in Table 2. To summarize, BNT score significantly increased over time in both groups (F(2,34)=15,231, p<0.001) while other scores remained stable (all p>0.05). Comparison between stroke groups and matched healthy participants were performed using two-sample t-tests as summarized in Table 3. Pre-scanning tests (BNT, SFT, COWAT, PPVT) showed significant differences between healthy participants and stroke participants for every time-point. Behavioral tests related to MRI scanning (SD, TD, noun recall – post VG testing) showed no significant difference between healthy participants and stroke participants except for TD.
Table 2.
Behavioral score statistics for stroke participants. Repeated-measures ANOVAs were computed for each score with session (2 sessions for Mini-CAL and 3 sessions for all other scores) and group (control vs. trained) as factors. Means and standard deviations (SD) are also provided for each test and each time point.
| BNT | SFT | COWAT | PPVT | SD | TD | Noun rec | Mini-CAL | ||
|---|---|---|---|---|---|---|---|---|---|
| Time | F-value | 15.231 | 1.575 | 1.6 | 0.487 | 1.138 | 0.558 | 0.655 | 2.226 |
| p-value | <0.001 | 0.221 | 0.216 | 0.618 | 0.332 | 0.577 | 0.525 | 0.155 | |
| Time X Group | F-value | 0.305 | 1.509 | 2.256 | 0.016 | 0.597 | 0.093 | 1.046 | 2.102 |
| p-value | 0.738 | 0.235 | 0.12 | 0.983 | 0.556 | 0.911 | 0.362 | 0.166 | |
| Trained | Mean 1 | 33.13 | 13.63 | 5.38 | 197.38 | 13.13 | 12.81 | 0.80 | 45.25 |
| SD 1 | 18.93 | 9.05 | 2.77 | 16.10 | 3.99 | 10.91 | 0.12 | 13.57 | |
| Mean 2 | 36.13 | 13.38 | 5.38 | 196.25 | 10.69 | 14.31 | 0.75 | N/A | |
| SD 2 | 19.61 | 7.07 | 2.97 | 14.64 | 6.03 | 3.70 | 0.20 | N/A | |
| Mean 3 | 37.50 | 14.38 | 5.13 | 197.88 | 14.25 | 14.13 | 0.78 | 0.78 | |
| SD 3 | 17.87 | 8.68 | 3.44 | 14.92 | 7.94 | 6.74 | 0.17 | 0.17 | |
| Untrained | Mean 1 | 31.82 | 19.27 | 8.09 | 200.00 | 13.05 | 15.73 | 0.82 | 47.09 |
| SD 1 | 18.98 | 12.11 | 6.32 | 16.43 | 9.20 | 7.58 | 0.12 | 16.48 | |
| Mean 2 | 36.00 | 22.55 | 9.55 | 198.82 | 13.14 | 16.50 | 0.83 | N/A | |
| SD 2 | 17.93 | 12.61 | 6.90 | 19.37 | 8.23 | 8.06 | 0.10 | N/A | |
| Mean 3 | 37.55 | 21.73 | 11.00 | 201.09 | 13.86 | 17.68 | 0.86 | 0.86 | |
| SD 3 | 16.69 | 9.91 | 7.67 | 14.33 | 8.28 | 7.74 | 0.12 | 0.12 |
Table 3.
Comparison of behavioral tests results between healthy and stroke participants. Two-sample t-tests were computed to test for differences between each stroke group and matched healthy participants.
| BNT | SFT | COWAT | PPVT | SD | TD | Noun rec | ||
|---|---|---|---|---|---|---|---|---|
| Healthy group (control) | Mean | 56.5 | 51.68 | 36.31 | 216.43 | 16.81 | 26.37 | 0.72 |
| SD | 3.61 | 9.61 | 11.85 | 7.64 | 2.76 | 6.5 | 0.15 | |
| Healthy group (Trained) | Mean | 56.22 | 58.59 | 37.18 | 213.95 | 16.59 | 26.04 | 0.73 |
| SD | 5.41 | 12.52 | 9.94 | 11.9 | 2.36 | 4.91 | 0.13 | |
| T-tests between stroke groups and healthy group (p-values) | Control 1 | <0.001 | <0.0001 | <0.0001 | <0.001 | 0.0061 | <0.001 | 0.21 |
| Control 2 | <0.001 | <0.0001 | <0.0001 | <0.001 | 0.0052 | <0.001 | 0.71 | |
| Control 3 | <0.001 | <0.0001 | <0.0001 | <0.001 | 0.2261 | <0.001 | 0.46 | |
| Trained 1 | <0.001 | <0.0001 | <0.0001 | 0.0089 | 0.0385 | <0.001 | 0.06 | |
| Trained 2 | <0.001 | <0.0001 | <0.0001 | 0.0091 | 0.0258 | 0.0011 | 0.03 | |
| Trained 3 | <0.001 | <0.0001 | <0.0001 | 0.01 | 0.0086 | <0.001 | 0.008 |
FMRI results
In order to improve potential reproducibility, the threshold for every fMRI group statistical analysis was set to p<0.01 uncorrected with 50 contiguous voxels. Such analyses are preferred by some and justified in order to not miss the true effects by applying overly conservative thresholds to avoid type I errors [44]. The view of these authors is that such errors, if present, are “self-erasing” as they will not be replicated in subsequent studies. This is particularly true with studies of a population where inter-individuals’ differences are large, such as patients with stroke. Thus, while the selected thresholds were lenient, they were used to assess the potential for cortical plasticity and to provide a springboard for future studies and analyses.
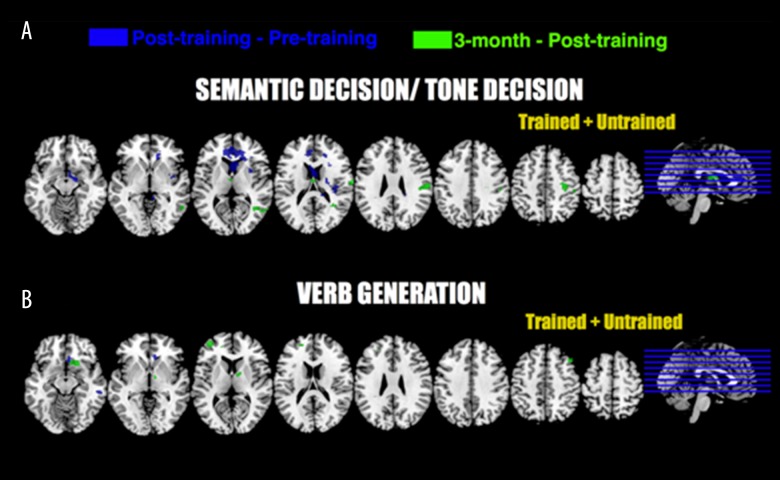
Using SPM12 second level flexible factorial algorithm, a repeated-measure ANOVA was conducted on fMRI data on a voxel-wise basis (SDTD and VG separately) with groups (trained and untrained) and sessions (before training, after training and three-month follow-up) as factors. No differences in fMRI signal related to any factor were found to be significant. Two sample t-tests were computed between each stroke group (trained and untrained) and healthy controls for each session and each task. Results for comparisons between stroke groups (trained versus untrained) are provided in Figure 2; peak coordinates and locations are provided in Table 4. Results for comparisons between stroke groups and healthy controls are provided in Figure 3; peak coordinates and locations are provided in Table 5.
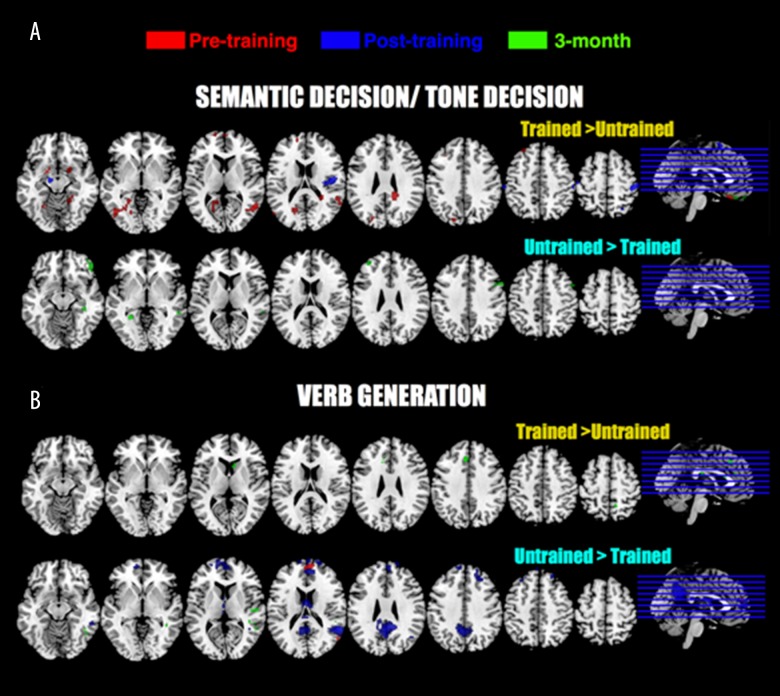
Figure 2.
Functional MRI tasks results (GLM analyses). Two-sample t-tests were computed between trained and untrained stroke group for each task (SD/TD and VG) and each session. For every contrast, uncorrected data are provided (p<0.01, 50 contiguous voxels). Peak coordinates are provided in Table 4. All pictures are in neurological convention.
Table 4.
Main peak coordinates of fMRI two sample t-test results for comparison between trained and untrained group as depicted in Figure 2.
| Semantic decision | Verb generation | ||||||
|---|---|---|---|---|---|---|---|
| Coordinates (x y z) | BA | T-value | Coordinates (x y z) | BA | T-value | ||
| Trained > Untrained | Pre | [52; −60; 10] | 39 | 5.54 | [8; −52; 54] | 7 | 3.6 |
| [−20; −52; 4] | 30 | 5.27 | |||||
| [−18; 2; −14] | 34 | 4.98 | |||||
| [−50; −76; 24] | 39 | 4.23 | |||||
| [−26; 36; 44] | 8 | 3.93 | |||||
| [0; 32; −26] | 11 | 3.78 | |||||
| [60; −2; −18] | 21 | 3.72 | |||||
| [40; 22; −24] | 38 | 3.42 | |||||
| [20; 4; −12] | 34 | 3.19 | |||||
| [6; 62; 4] | 10 | 3.18 | |||||
| Post | [44; −26; 58] | 3 | 3.97 | None | |||
| [48; −12; 16] | 43 | 3.87 | |||||
| [10; −12; 62] | 6 | 3.83 | |||||
| [−58; −24; 52] | 2 | 3.80 | |||||
| [−12; −18; −16] | 28 | 3.62 | |||||
| [24; −56; 66] | 7 | 3.59 | |||||
| [−38; −46; 64] | 5 | 3.25 | |||||
| 3M | [−20; −12; −22] | 47 | 6.18 | [−4; 26; 40] | 8 | 3.84 | |
| [−34; −44; 64] | 5 | 5.19 | [−4; −22; 22] | 23 | 3.60 | ||
| [−44; 22; −32] | 38 | 4.18 | [8; 20; 10] | Caudate | 3.42 | ||
| [2; 40; −26] | 11 | 3.97 | |||||
| Untrained > Trained | Pre | None | [56; −68; 14] | 39 | 4.41 | ||
| [32; 8; −34] | 38 | 4.03 | |||||
| [4; 56; 20] | 10 | 3.99 | |||||
| [−66; −18; −24] | 20 | 3.97 | |||||
| Post | None | [20; 64; 24] | 10 | 6.98 | |||
| [56; −52; 16] | 22 | 6.67 | |||||
| [−8; −54; 36] | 7 | 5.60 | |||||
| [24; 38; 44] | 8 | 4.42 | |||||
| [58; −2; −32] | 20 | 4.26 | |||||
| [68; −36; −6] | 21 | 3.73 | |||||
| [−26; 34; 46] | 8 | 3.61 | |||||
| 3M | [−28; −46; −2] | 19 | 4.83 | [54; −18; 10] | 41 | 3.80 | |
| [50; 40; −10] | 47 | 4.07 | [48; −58; −8] | 19 | 3.46 | ||
| [−36; 44; 32] | 9 | 3.97 | [50; −40; 10] | 21 | 3.36 | ||
| [60; 14; 34] | 9 | 3.63 | [−64; −18; −30] | 20 | 3.35 | ||
| [60; −38; 6] | 22 | 3.27 | |||||
Coordinates are in Talairach space, BA – Brodmann area.
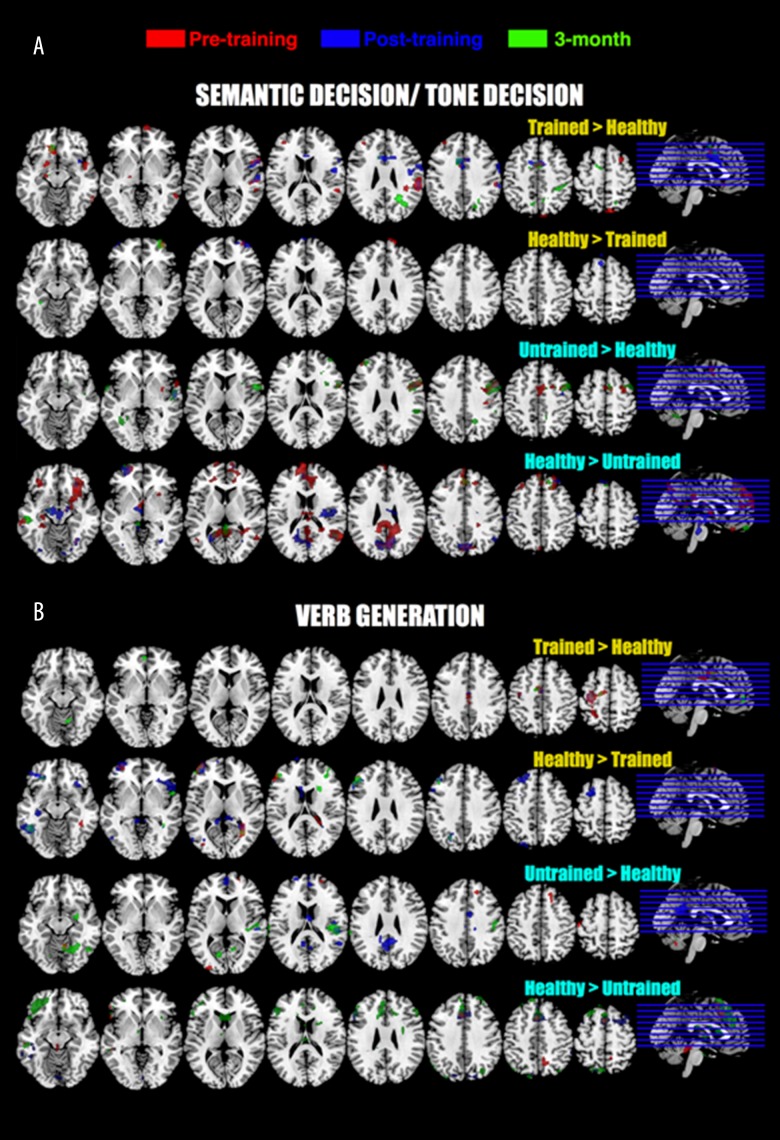
Figure 3.
Functional MRI tasks results (GLM analyses). Two-sample t-tests were computed between trained and healthy group and between untrained and healthy group for each task (SD/TD and VG) and each session. For every contrast, only uncorrected contrasts are provided (p<0.01, 50 contiguous voxels). Peak coordinates are provided in Table 5. All pictures are in neurological convention.
Table 5A.
Main peak coordinates of fMRI two sample t-test results for comparison between trained and healthy control group as depicted in Figure 3.
| Semantic decision | Verb generation | ||||||
|---|---|---|---|---|---|---|---|
| Coordinates (x y z) | BA | T-value | Coordinates (x y z) | BA | T-value | ||
| Trained > Healthy | Pre | [−8; 28; −12] | 11 | 4.69 | [−34; −20; 58] | 4 | 4.90 |
| [−16; 6; −14] | 34 | 4.29 | [−26; −48; 60] | 7 | 3.21 | ||
| [56; −36; 28] | 40 | 4.16 | [10; −48; −24] | Cerebellum | 3.01 | ||
| [62; 12; 4] | 44 | 3.70 | |||||
| [28; 12; 60] | 6 | 3.65 | |||||
| [6; 6; 44] | 32 | 3.38 | |||||
| [60; −48; −6] | 37 | 3.33 | |||||
| [−18; −16; −10] | 28 | 3.23 | |||||
| [50; 4; −12] | 38 | 3.18 | |||||
| Post | [−12; 8; 44] | 24 | 4.22 | [−34; −18; 60] | 7 | 3.87 | |
| [60; 8; 10] | 44 | 4.16 | [−4; −8; 46] | 24 | 3.12 | ||
| [52; 2; 12] | 6 | 3.41 | |||||
| [60; −18; 8] | 42 | 3.46 | |||||
| [64; −34; 36] | 40 | 3.44 | |||||
| [38; 8; −8] | 13 | 3.33 | |||||
| 3M | [−8; 30; −14] | 11 | 4.15 | [−4; −4; 44] | 24 | 3.48 | |
| [−12; 8; 40] | 32 | 4.01 | [−32; −18; 58] | 4 | 3.19 | ||
| [−18; −18; −8] | 35 | 3.38 | [−4; 50; −4] | 10 | 3.05 | ||
| [32; −38; 46] | 40 | 3.16 | |||||
| Healthy > Trained | Pre | [10; 68; 28] | 10 | 3.49 | [30; −62; 10] | 31 | 4.28 |
| [40; −48; −8] | 37 | 4.07 | |||||
| [−36; 56; 4] | 10 | 3.77 | |||||
| [−6; 8; 70] | 6 | 3.08 | |||||
| Post | [−4; 70; 14] | 10 | 3.89 | [−34; 40; −10] | 11 | 4.94 | |
| [−6; 26; 58] | 6 | 3.60 | [−68; −44; −8] | 21 | 4.85 | ||
| [−24; −40; −20] | Cerebellum | 3.36 | [−22; 10; 56] | 6 | 4.60 | ||
| [42; 58; 4] | 10 | 3.35 | [−44; 30; 22] | 46 | 4.32 | ||
| [−42; 58; 2] | 10 | 3.12 | [−14; 70; 18] | 10 | 4.24 | ||
| [−34; −72; 12] | 19 | 3.35 | |||||
| [−22; −66; 38] | 7 | 3.34 | |||||
| [−8; 16; 20] | Cerebellum | 3.32 | |||||
| [34; 24; −10] | 47 | 3.23 | |||||
| 3M | [36; 10; −30] | 9 | 3.77 | [−30; 28; −16] | 47 | 3.90 | |
| [34; 58; −2] | 10 | 3.43 | [−48; −50; −10] | 37 | 3.85 | ||
| [14; −40; −20] | Cerebellum | 3.30 | [−50; 28; 30] | 9 | 3.47 | ||
| [−16; −36; −20] | Cerebellum | 3.18 | [−22; −66; 36] | 7 | 3.26 | ||
| [32; −58; 8] | 30 | 3.13 | |||||
| [38; 36; 14] | 46 | 3.07 | |||||
| [54; 8; −4] | 22 | 2.93 | |||||
Coordinates are in Talairach space, BA – Brodmann area.
Table 5B.
Main peak coordinates of fMRI two sample t-test results for comparison between untrained and healthy control group as depicted in Figure 3.
| Semantic decision | Verb generation | ||||||
|---|---|---|---|---|---|---|---|
| Coordinates (x y z) | BA | T-value | Coordinates (x y z) | BA | T-value | ||
| Untrained > Healthy | Pre | [56; 10; 30] | 9 | 5.62 | [24; −42; −32] | Cerebellum | 4.03 |
| [10; 2; 56] | 6 | 3.65 | [−30; −38; 72] | 5 | 3.99 | ||
| [−40; 48; 28] | 9 | 3.22 | [−48; −18; 58] | 3 | 3.89 | ||
| [38; −22; 38] | 3 | 3.18 | [−28; −44; −38] | Cerebellum | 3.52 | ||
| [18; 32; 50] | 8 | 3.2 | |||||
| [24; 58; 10] | 10 | 3.08 | |||||
| [−28; −94; 8] | 18 | 3.07 | |||||
| Post | [54; 10; 36] | 9 | 4.41 | [20; −46; −34] | Cerebellum | 4.55 | |
| [14; 16; −24] | 47 | 3.57 | [−22; 60; 20] | 10 | 4.22 | ||
| [−58; −56; −6] | 37 | 3.53 | [50; −30; 12] | 41 | 4.13 | ||
| [56; −6; −2] | 22 | 3.45 | [2; −50; 28] | 31 | 4.05 | ||
| [2; −2; 40] | 24 | 3.49 | |||||
| [2; 52; 14] | 10 | 3.45 | |||||
| [54; −50; 16] | 22 | 3.42 | |||||
| [16; −26; 34] | 31 | 3.12 | |||||
| 3M | [54; 10; 36] | 9 | 6.85 | [10; −52; −18] | cerebellum | 4.47 | |
| [12; 4; 54] | 6 | 4.84 | [34; −28; 14] | 13 | 4.44 | ||
| [16; −54; 40] | 7 | 4.37 | [−68; −34; 24] | 40 | 4.24 | ||
| [−36; 46; 32] | 9 | 4.02 | [−42; −16; −32] | 20 | 4.03 | ||
| [4; 16; −28] | 11 | 3.83 | [−30; −38; 70] | 5 | 3.97 | ||
| [−60; 4; 4] | 22 | 3.82 | [20; 28; −18] | 11 | 3.79 | ||
| [12; −48; 52] | 7 | 3.72 | [−14; −70; 8] | 23 | 3.46 | ||
| [8; −56; −30] | Cerebellum | 3.59 | [46; −20; 36] | 3 | 3.19 | ||
| [26; 28; 22] | 32 | 3.42 | [12; −60; 4] | 18 | 3.17 | ||
| [34; 12; 18] | 13 | 3.3 | |||||
| Heathy > Untrained | Pre | [30; 22; −10] | 47 | 6.31 | [−46; 22; −6] | 47 | 4.8 |
| [58; −56; 16] | 22 | 6.07 | [−2; 18; 44] | 6 | 4.31 | ||
| [−6; 62; 18] | 10 | 5.24 | [−40; −32; −24] | Cerebellum | 3.68 | ||
| [−32; 18; −16] | 47 | 4.85 | [10; −58; 48] | 7 | 3.43 | ||
| [−38; 24; 50] | 8 | 4.3 | [−2; −38; −16] | Cerebelllum | 3.27 | ||
| [0; 30; −28] | 32 | 4.29 | |||||
| [34; −60; −8] | Cerebellum | 3.87 | |||||
| [−40; 4; −42] | 20 | 3.81 | |||||
| [30; −10; −38] | 20 | 3.72 | |||||
| [−48; −76; 26] | 39 | 3.66 | |||||
| [−60; −28; −14] | 21 | 3.42 | |||||
| Post | [−12; −18; −16] | 28 | 6.04 | [0; 14; 52] | 6 | 10.52 | |
| [4; −74; 30] | 7 | 5.03 | [−60; −24; −30] | 20 | 5.25 | ||
| [32; −62; −8] | Cerebellum | 4.59 | [−62; −48; −22] | 37 | 4.98 | ||
| [68; −8; −18] | 21 | 4.57 | [18; −66; −38] | Cerebellum | 4.86 | ||
| [−26; 60; −2] | 10 | 4.3 | [−54; 18; 24] | 9 | 4.85 | ||
| [8; −12; 24] | 23 | 4.24 | [−22; 6; −44] | 38 | 4.3 | ||
| [−30; 18; −18] | 47 | 4.04 | [60; −42; 46] | 40 | 3.82 | ||
| [26; 38; 48] | 8 | 3.97 | [4; −86; −10] | 18 | 3.76 | ||
| [16; −6; −16] | 34 | 3.87 | [−32; 8; 62] | 6 | 3.74 | ||
| [32; 32; −8] | 47 | 3.73 | [18; −84; 38] | 19 | 3.62 | ||
| [−4; 44; 16] | 9 | 3.67 | [−18; −72; 58] | 7 | 3.33 | ||
| [−38; −46; 66] | 5 | 3.57 | [−30; −82; 40] | 19 | 3.21 | ||
| [−46; −28; 40] | 2 | 3.57 | |||||
| [54; −62; 40] | 39 | 3.54 | |||||
| [−2; 36; 54] | 8 | 3.52 | |||||
| 3M | [−18; −14; −20] | 28 | 5.26 | [−2; 16; 50] | 8 | 6.32 | |
| [−58; −8; −28] | 20 | 4.91 | [−46; 24; −6] | 47 | 5.64 | ||
| [−54; −24; −14] | 21 | 4.33 | [−10; −24; 24] | Caudate | 4.31 | ||
| [−44; 24; −30] | 38 | 4.06 | [34; −66; −32] | Cerebellum | 4.24 | ||
| [−8; 56; −28] | 11 | 3.94 | [30; 54; 36] | 9 | 4.23 | ||
| [0; 38; 38] | 8 | 3.84 | [56; 28; 24] | 46 | 4.1 | ||
| [−6; −40; −8] | Cerebellum | 3.79 | [30; 24; −8] | 47 | 3.71 | ||
| [58; 8; −24] | 21 | 3.79 | [−16; −86; 46] | 19 | 3.63 | ||
| [52; −56; 14] | 22 | 3.77 | [−18; −68; 54] | 7 | 3.52 | ||
| [0; −50; 8] | 29 | 3.58 | [30; 0; 24] | 13 | 3.49 | ||
| [−18; −52; 8] | 30 | 3.53 | |||||
Coordinates are in Talairach space, BA – Brodmann area.
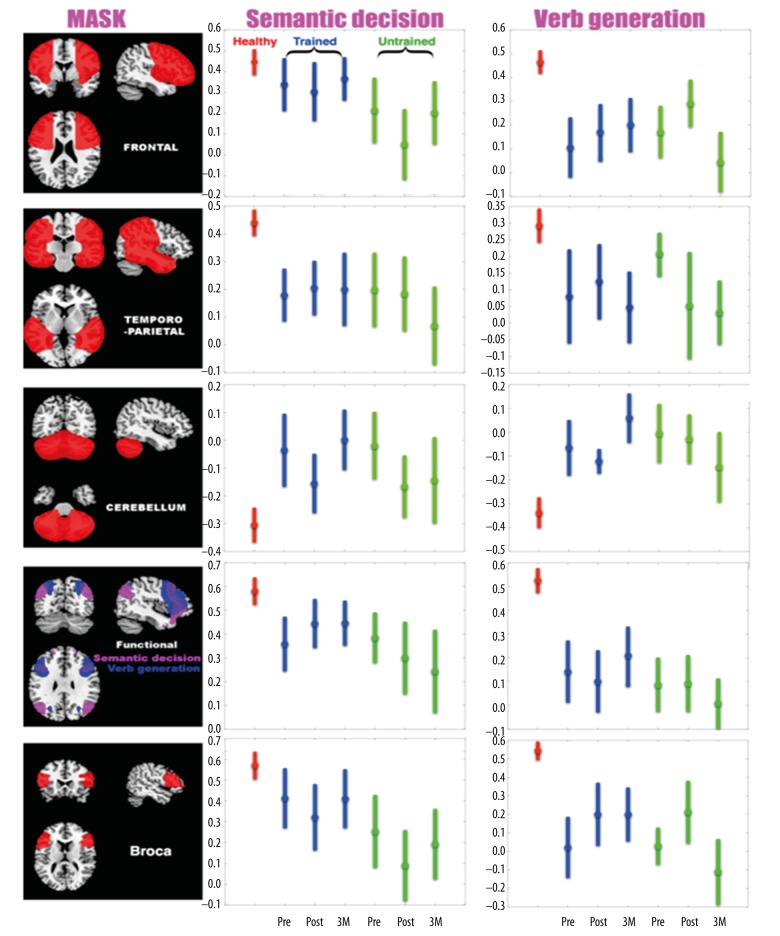
Laterality index results
Laterality indices were computed for each participant, each fMRI, each session, and each region of interest (five different masks, see Material and Methods section). Results are depicted in Figure 4. Repeated-measures ANOVAs on LI values for both stroke groups revealed no significant effect of training. For each region of interest (five different masks) and each task a Group X Time-point ANOVA was computed with two groups (trained versus untrained) and three time-points (pre-training, post-training and three-month follow-up). ANOVA results are provided in Table 6.
Figure 4.
Lateralization Index (LI) results. Two-sample t-tests revealed no significant effect of training. LI values for healthy group are consistently different. Healthy group LI values are depicted in red, trained group LI values are depicted in blue, untrained group LI values are depicted in green. Error bars represent standard error of the mean. Pre- refers to pre-training time-point, post- refers to post-training time-point, and 3M refers to 3-month follow-up.
Table 6.
Repeated-measures ANOVAs on LI values for both stroke groups. For each region of interest (5 different masks) and each task a Group X Time-point ANOVA was computed with two groups (trained vs. untrained) and three time-points.
| Frontal | Temp-Par | Cerebellum. | GLM | Broca’s | |||
|---|---|---|---|---|---|---|---|
| Semantic decision | Time | F-value | 0.81 | 0.25 | 1.21 | 0.05 | 0.66 |
| p-value | 0.45 | 0.77 | 0.31 | 0.95 | 0.51 | ||
| Time X Group | F-value | 0.25 | 0.35 | 0.49 | 0.73 | 0.05 | |
| p-value | 0.77 | 0.7 | 0.61 | 0.48 | 0.94 | ||
| Verb Generation | Time | F-value | 0.84 | 0.25 | 0.11 | 0.02 | 1.8 |
| p-value | 0.43 | 0.77 | 0.88 | 0.97 | 0.18 | ||
| Time X Group | F-value | 1.3 | 0.35 | 1.8 | 0.65 | 1.54 | |
| p-value | 0.28 | 0.7 | 0.18 | 0.52 | 0.22 |
Regression analyses
As BNT score was the only behavioral measurement showing a significant change over time in stroke patients (Table 2); regression analyses were computed between fMRI results and BNT scores as follows. Difference of cortical activity between post-treatment time-point and pre-treatment time-point was regressed with difference over time in BNT score (same time-points). A similar regression was also computed between three-month time-point and post-treatment time-point. Those regressions were computed in order to find the cortical areas associated with improvement in BNT scores over time. Results for those regressions analyses are depicted in Figure 5; peak coordinates and locations are provided in Table 7. Areas associated with BNT improvement between pre- and post-treatment are anterior cingulate, thalamus, cerebellum, and posterior cingulate for SDTD, anterior cingulate and temporal gyrus for VG, all located in right hemisphere.
Figure 5.
Functional MRI tasks results of regression analyses with BNT (p<0.01; 50 contiguous voxels). Functional difference over time for each task is regressed with difference in BNT score over time. Peak coordinates are provided in Table 7. All pictures are in neurological convention.
Table 7.
Main peaks coordinates of regression analyses between fMRI results of both stroke groups (Trained and Untrained) as depicted in Figure 5 and BNT behavioral scores.
| Semantic decision | Verb generation | ||||||
|---|---|---|---|---|---|---|---|
| Coordinates (x y z) | Brodman | T-value | Coordinates (x y z) | Brodman | T-value | ||
| Trained + Untrained | Post minus Pre | [8; 32; 12] | 24 | 5.25 | [6; 28; −10] | 32 | 5.21 |
| [16; −8; −12] | 28 | 4.50 | [58; −32; −16] | 20 | 3.82 | ||
| [22; −14; 14] | Thalamus | 4.44 | |||||
| [6; −42; −6] | Cerebellum | 3.88 | |||||
| [32; 4; −6] | Cerebellum | 3.65 | |||||
| [22; −34; 26] | 31 | 3.58 | |||||
| [34; 16; 14] | 13 | 3.09 | |||||
| 3M minus Post | [52; −64; 6] | 37 | 4.13 | [46; 22; 44] | 8 | 4.16 | |
| [66; −12; 18] | 43 | 3.95 | [−38; 52; 10] | 10 | 3.60 | ||
| [34; −20; 48] | 4 | 3.59 | [20; 16; −12] | 47 | 3.25 | ||
| [2; −10; 14] | Thalamus | 3.33 | [−20; 48; 22] | 19 | 3.05 | ||
Coordinates are in Talairach space, BA – Brodmann area.
Areas associated with BNT improvement between three-month and post-treatment were right temporal gyrus, right postcentral, and precentral gyri, and thalamus for SDTD, and left middle and superior frontal gyri for VG.
Discussion
The aim of the present study was to examine the effects of intensive language training (CIAT) on the cortical correlates of post-stroke recovery in patients with chronic (> one year) post-stroke aphasia who participated in a randomized trial of CIAT [7] and who were able to receive fMRI. Behavioral results revealed a relatively small effect of CIAT compared to observation only (untrained group) on naming performance. Although patients in the CIAT group numerically outperformed the observation group during the post-intervention testing, these differences were not statistically different but were in the expected direction [7]. Similarly, fMRI comparison between trained and untrained groups revealed only relatively minor effects of CIAT training over time on cortical activity (Figure 2). There may be several possible explanations for the relative lack of change observed in our data, including those eluded to in our original manuscript [7] that relate to very high variability in the studied cohort. In addition, other possibilities include the fact that CIAT is not associated with significant changes in cortical activity. Indeed, more than a year after stroke, spontaneous recovery is known to be very unlikely and little is known about the possibility of treatment-related improvements after 12 months [5]. However, this explanation is unlikely given that other studies observed neuroimaging changes in response to other types of therapy [45,46]. Thus, another and more plausible explanation for the relative lack of differences between groups is the substantial variability in the collected neuroimaging data (Figure 2) suggesting that a larger sample of individuals needs to be collected in order to determine the presence or absence of significant effects of the intervention [7]. This is discussed in more detail in the following section.
No significant Group X Time interactions were found between trained and untrained groups. The possible, and in fact likely, explanation for this relative lack of statistically significant differences between groups is the high variability among patients in lesion size and location as well as in behavioral performance. Two-sample t-tests between untrained and trained groups showed results consistent with this assumption (Figure 2). Moreover, SDTD and VG tasks resulted in somewhat different activation patterns. The trained group had higher cortical activity pre- and immediately post-training for SDTD (untrained group for VG) and untrained group exhibits higher cortical activity at three-months for SDTD (trained group for VG). Because these fMRI language tasks examine different aspects of language (semantic decision versus verb generation), this suggests that CIAT training may have different effects on different aspects of language. Comparisons between stroke patients and healthy controls (Figure 3) support the idea that the observed results may be related to high variability in the stroke data: two main observations can be made concerning the SDTD results. First, at three-months, the untrained group had more activity in the right frontal area (Broca area homologue) compared to healthy participants. This is consistent with the previously observed spontaneous but inefficient reorganization of language function after stroke to the right (non-dominant) hemisphere [47–50]. However, this effect was not observed in the trained group, suggesting that changes in language lateralization observed in this study, while not significant, may be characterized as shifts of the fMRI activation patterns to the previously dominant for language left hemisphere. This is consistent with previous studies that showed temporal changes in post-stroke language recovery and the need for increased left hemispheric participation in order for the language functions to return as close to pre-stroke level as possible [1,51,52]. This idea of a reorganization of language function after stroke to the right (non-dominant) hemisphere is also supported by the results from the regression analyses: a vast majority of cortical areas correlated with BNT score improvement over time was located in the right hemisphere (Figure 5, Table 7). Another possibility is that the observed reorganization to the right hemisphere might not be related to language function recovery, but, as it has been suggested previously, may even inhibit the recovery [53–58]. Second, there appears to be a relatively minimal difference in fMRI activity between healthy participants compared to trained or untrained participants (Figure 2, SDTD: healthy > trained and healthy > untrained). This lack of differences is likely related to the inter-individual variability within stroke patients rather than other reasons. It appears, at least in our cohort, that CIAT training does not reduce this variability.
In general, the results of the VG task appeared to be quite different than the results of the SDTD task. The majority of differences between healthy participants and stroke participants appeared to arise after the training time-point (post-training and three-months) and those differences were of greater magnitude in the trained group (Figure 3). This suggests that CIAT training might promote the functional reorganization of language functions following a stroke. Differences between SDTD and VG results were also found for regression analyses between BNT behavioral score and fMRI results. Areas associated with BNT improvement between pre- and post-treatment were anterior cingulate, thalamus, cerebellum, and cingulate for SDTD, anterior cingulate and temporal gyrus for VG, all located in right hemisphere. Areas associated with BNT improvement between three-months and post-treatment were temporal gyrus, postcentral gyrus, precentral gyrus, and thalamus for SDTD (all in right hemisphere), and left middle and superior frontal gyri for VG.
Overall, the differences in fMRI between healthy controls and stroke patients (trained and untrained) suggest that stroke affects the organization of different language functions in a different manner, as documented by the differences between VG and SDTD results including regression analyses of the language tasks with BNT (Figure 5, Table 7). Future studies should be careful in the interpretation of their results concerning the reorganization of language brain functions following a stroke (or any other brain injury) as the observed differences could be, in part, related to the type of the language task used [33]. The language lateralization results appear to be consistent with this idea. While the results of language lateralization in stroke patients were clearly different when compared to healthy controls, there were no differences between the stroke patients at various time points despite the fact that there were some differences in the activation patterns (Figure 3). This may indicate that language lateralization is a unidimensional measure that does not reflect the complexities of functional reorganization of language functions in the brain that are more complex than simple left/right hemisphere balance and potential compensation by right homotopic areas, as suggested previously [59,60]. Despite the vast majority of differences in results between SDTD and VG task, a cortical area correlated with behavioral score improvement over time was found for both fMRI tasks. This area was the anterior cingulate, which has been described as having a central role in processing and regulating information in the human brain [61,62]. In this case, we believe that the anterior cingulate played a critical role in regulating information in order to compensate for functional loss of language functions following stroke.
It is well known that aging has a deep impact on the human brain. Age-related decline in cerebral macrostructure, such as reductions in gray and white matter volume, is well-documented [63–67]. Those age-related differences in cortical structure are likely to have a deep negative impact on cognitive functioning [68] and, therefore, potential for cortical plasticity [69]. Moreover, such a traumatic event as a left ischemic stroke further alters cognitive functions and, thus, lowers substantially potential plasticity. Altogether, it is possible that CIAT training alone may not be sufficient to induce improvement compared to spontaneous recovery when training starts more than 12 months post-stroke and other supportive measures, such as a transfer package, may be needed [70]. Future studies should explore the possibility of enhancing the effects of language interventions by associating it with other forms of interventions that may exert effects on cognitive functions via different mechanisms such as: physical exercise [71], transcranial direct current stimulation (tDCS) or transcranial magnetic stimulation [72,73].
Limitations to the current study should also be considered. As indicated earlier, the potential caveat of group analysis in stroke patients are the inter-individual differences. Brain areas affected by strokes are different (even if roughly located in same areas) and even if they lead to the same language challenges and type of aphasia, the impact on brain activity is very likely to be different across individuals. This likely explains the large standard errors obtained in stroke patients’ behavioral scores and lateralization indices (Figure 3). Also, this study was intended to be preliminary in nature. As such, this was reflected in our approach to the analyses and computation of various correlations over extensive brain areas (LI masks for example). While these analyses and results provide important information, the analytic approach may have had a negative impact on the power of this study. Thus, all results presented here should be considered as framework for future explorations.
Conclusions
Constraint-induced aphasia therapy is an attempt to adapt a successful therapeutic approach to a different functional modality. The changes in cortical activation patterns between pre- and post-intervention scanning sessions and the observed trends in behavioral data suggest that despite common cortical areas associated with behavioral improvement, variability among people suffering from stroke-induced aphasia is so important that trainings such as CIAT have a variable impact as well. Studies that include larger participant samples are needed to assess the effects of CIAT on post-stroke language recovery.
Footnotes
Source of support: Departmental sources
References
- 1.Saur D, Hartwigsen G. Neurobiology of language recovery after stroke: Lessons from neuroimaging studies. Arch Phys Med Rehabil. 2012;93:S15–25. doi: 10.1016/j.apmr.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PM, Jørgensen HS, Nakayama H, et al. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann Neurolm. 1995;38:659–66. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 3.Carlomagno S, Pandolfi M, Labruna L, et al. Recovery from moderate aphasia in the first year poststroke: Effect of type of therapy. Arch Phys Med Rehabil. 2001;82:1073–80. doi: 10.1053/apmr.2001.25155. [DOI] [PubMed] [Google Scholar]
- 4.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41:172–87. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 5.Berthier ML, Pulvermüller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol. 2011;7:86–97. doi: 10.1038/nrneurol.2010.201. [DOI] [PubMed] [Google Scholar]
- 6.André J-M, Didier J-P, Paysant J. “Functional motor amnesia” in stroke (1904) and “learned non-use phenomenon” (1966)”. J Rehabil Med. 2004;36:138–40. doi: 10.1080/16501970410026107. [DOI] [PubMed] [Google Scholar]
- 7.Szaflarski JP, Ball AL, Vannest J, et al. Constraint-induced aphasia therapy for treatment of chronic post-stroke aphasia: A randomized, blinded, controlled pilot trial. Med Sci Monit. 2015;21:2861–69. doi: 10.12659/MSM.894291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulvermüller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32(7):1621–26. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 9.Szaflarski JP, Ball A, Grether S, et al. Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke. Med Sci Monit. 2008;14:CR243–250. [PMC free article] [PubMed] [Google Scholar]
- 10.Meinzer M, Djundja D, Barthel G, et al. Long-term stability of improved language functions in chronic aphasia after constraint-induced aphasia therapy. Stroke. 2005;36:1462–66. doi: 10.1161/01.STR.0000169941.29831.2a. [DOI] [PubMed] [Google Scholar]
- 11.Maher LM, Kendall D, Swearengin JA, et al. A pilot study of use-dependent learning in the context of Constraint Induced Language Therapy. J Int Neuropsychol Soc. 2006;12:843–52. doi: 10.1017/S1355617706061029. [DOI] [PubMed] [Google Scholar]
- 12.Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: A new family of techniques with broad application to physical rehabilitation – a clinical review. J Rehabil Res Dev. 1999;36:237–51. [PubMed] [Google Scholar]
- 13.Meinzer M, Flaisch T, Breitenstein C, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–46. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Meinzer M, Elbert T, Wienbruch C, et al. Intensive language training enhances brain plasticity in chronic aphasia. BMC Biol. 2004;2:20. doi: 10.1186/1741-7007-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulvermüller F, Hauk O, Zohsel K, et al. Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. Neuroimage. 2005;28:481–89. doi: 10.1016/j.neuroimage.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Richter M, Miltner WHR, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131:1391–401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 17.Breier JI, Maher LM, Novak B, Papanicolaou AC. Functional imaging before and after constraint-induced language therapy for aphasia using magnetoencephalography. Neurocase. 2006;12(6):322–31. doi: 10.1080/13554790601126054. [DOI] [PubMed] [Google Scholar]
- 18.Breier JI, Juranek J, Maher LM, et al. Behavioral and neurophysiologic response to therapy for chronic aphasia. Arch Phys Med Rehabil. 2009;90:2026–33. doi: 10.1016/j.apmr.2009.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breier JI, Maher LM, Schmadeke S, et al. Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: A case study. Neurocase. 2007;13:169–77. doi: 10.1080/13554790701448200. [DOI] [PubMed] [Google Scholar]
- 20.Mohr B, Difrancesco S, Harrington K, et al. Changes of right-hemispheric activation after constraint-induced, intensive language action therapy in chronic aphasia: fMRI evidence from auditory semantic processing1. Front Hum Neurosci. 2014;8:1–15. doi: 10.3389/fnhum.2014.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–78. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Binder JR, Frost JA, Hammeke TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karunanayaka P, Schmithorst VJ, Vannest J, et al. A group independent component analysis of covert verb generation in children: A functional magnetic resonance imaging study. Neuroimage. 2010;51:472–87. doi: 10.1016/j.neuroimage.2009.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn LM, Dunn DM. Peabody picture vocabulary Test. 4th ed. NCS Pearson, Inc.; 2007. [Google Scholar]
- 27.Kaplan E, Goodglass H. Boston naming test. 2nd ed. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 28.Lezak M. Neuropsychological assessment. Oxford Univ Press; 1995. [Google Scholar]
- 29.Kozora E, Cullum CM. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. Clin Neuropsychol. 1995;9:313–20. [Google Scholar]
- 30.Kim KK, Karunanayaka P, Privitera MD, et al. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011;20:613–22. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szaflarski JP, Holland SK, Jacola LM, et al. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen SE, Fox PT, Posner MI, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–89. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 33.Allendorfer J, Kissela BBM, Holland SK, Szaflarski JP. Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit. 2012;18(3):CR135–37. doi: 10.12659/MSM.882518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szaflarski JP, Allendorfer J, Byars AW, et al. Age at stroke determines post-stroke language lateralization. Restor Neurol Neurosci. 2014;32:733–42. doi: 10.3233/RNN-140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allendorfer J, Lindsell CJ, Siegel M, et al. Females and males are highly similar in language performance and cortical activation patterns during verb generation. Cortex. 2012;48:1218–33. doi: 10.1016/j.cortex.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Friston KJ, Williams S, Howard R, et al. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 39.Szaflarski JP, Schmithorst VJ, Altaye M, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bigras C, Shear PK, Vannest J, et al. The effects of temporal lobe epilepsy on scene encoding. Epilepsy Behav. 2013;26:11–21. doi: 10.1016/j.yebeh.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilke M, Lidzba K. LI-tool: A new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Wilke M, Schmithorst VJ. A bootstrap approach for assessing lateralization in functional imaging data. 2006:10. [Google Scholar]
- 43.Griffis JC, Allendorfer J, Szaflarski JP. Voxel-based Gaussian naïve Bayes classification of ischemic stroke lesions in individual T1-weighted MRI scans. J Neurosci Methods. 2016;257:97–108. doi: 10.1016/j.jneumeth.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–28. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szaflarski JP, Page SJ, Kissela BM, et al. Cortical reorganization following modified constraint-induced movement therapy: A study of 4 patients with chronic stroke. Arch Phys Med Rehabil. 2006;87:1052–58. doi: 10.1016/j.apmr.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Page SJ, Szaflarski JP, Eliassen JC, et al. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382–88. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leff A, Crinion J, Scott S, et al. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol. 2002;51:553–58. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- 48.Musso M, Weiller C, Kiebel S, et al. Training-induced brain plasticity in aphasia. Brain. 1999;122(Pt 9):1781–90. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 49.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–98. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 50.Tillema J-M, Byars AW, Jacola LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105:99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szaflarski JP, Allendorfer J, Banks C, et al. Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restor Neurol Neurosci. 2013;31:347–60. doi: 10.3233/RNN-120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffis JC, Nenert R, Allendorfer JB, Szaflarski JP. Interhemispheric plasticity following intermittent theta burst stimulation in chronic poststroke aphasia. Neural Plast. 2016;2016:20–23. doi: 10.1155/2016/4796906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen HJ, Petersen SE, Linenweber MRR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–94. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 54.Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: A positron emission tomography activation study. Ann Neurol. 2001;50:620–29. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- 55.Blank SC, Bird H, Turkheimer F, Wise RJS. Speech production after stroke: The role of the right pars opercularis. Ann Neurol. 2003;54:310–20. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- 56.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: An open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Naeser MA, Martin PI, Baker EH, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22:29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: A follow-up investigation. Stroke. 2007;38:1286–92. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- 59.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–34. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 60.Crinion J, Leff AP. Recovery and treatment of aphasia after stroke: Functional imaging studies. Curr Opin Neurol. 2007;20:667–73. doi: 10.1097/WCO.0b013e3282f1c6fa. [DOI] [PubMed] [Google Scholar]
- 61.Margulies DS, Kelly AMC, Uddin LQ, et al. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 62.Allman JM, Hakeem A, Erwin JM, et al. The anterior cingulate cortex. Ann NY Acad Sci. 2006;935:107–17. [PubMed] [Google Scholar]
- 63.Hafkemeijer A, Altmann-Schneider I, de Craen AJM, et al. Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell. 2014;13:1068–74. doi: 10.1111/acel.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walhovd KB, Westlye LT, Amlien I, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–32. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44:1247–58. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 67.Madhavan KM, McQueeny T, Howe SR, et al. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res. 2014;1562:11–22. doi: 10.1016/j.brainres.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marstaller L, Williams M, Rich A, et al. Aging and large-scale functional networks: White matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience. 2015;290:369–78. doi: 10.1016/j.neuroscience.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 69.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 70.Johnson ML, Taub E, Harper LH, et al. An enhanced protocol for constraint-induced aphasia therapy II: A case series. Am J Speech Lang Pathol. 2014;23:60–72. doi: 10.1044/1058-0360(2013/12-0168). [DOI] [PubMed] [Google Scholar]
- 71.Bherer L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann NY Acad Sci. 2015;1337:1–6. doi: 10.1111/nyas.12682. [DOI] [PubMed] [Google Scholar]
- 72.Jones KT, Stephens JA, Alam M, et al. Longitudinal neurostimulation in older adults improves working memory. PLoS One. 2015;10:e0121904. doi: 10.1371/journal.pone.0121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah PP, Szaflarski JP, Allendorfer J, Hamilton RH. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front Hum Neurosci. 2013;7:888. doi: 10.3389/fnhum.2013.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]