Figure 1.
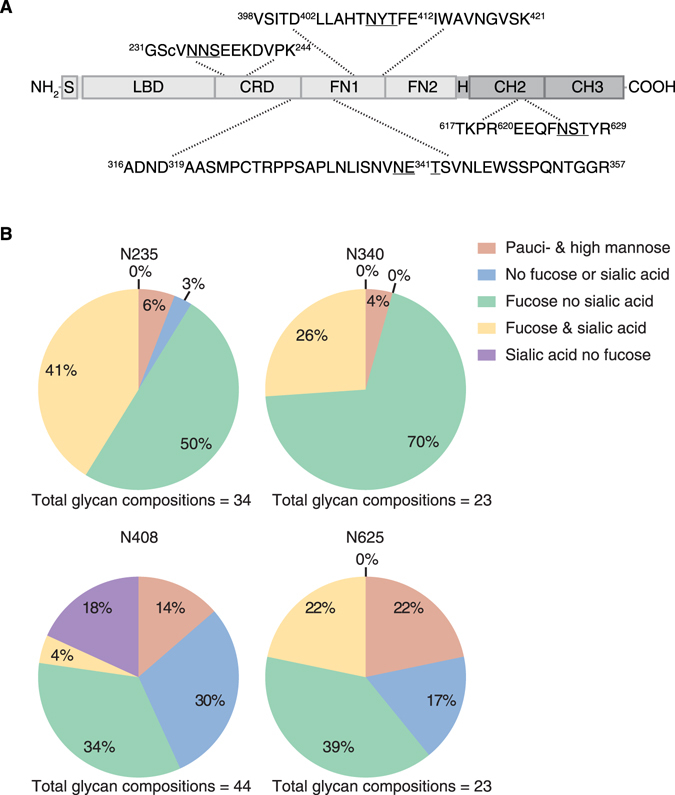
Glycosylation of wild type EphA4 Fc. (A) A schematic of EphA4 Fc (not to scale) identifying the EphA4 region (light grey) and the Fc region (dark grey). The EphA4 signal peptide (S) is located at the N-terminus of the protein and is followed by the ligand-binding domain (LBD), cysteine-rich domain (CRD) and two fibronectin type III repeats (FN1) and (FN2)4. The IgG4 Fc portion of the protein includes the hinge (H) region and the CH2 and CH3 constant immunoglobulin domains. Observed tryptic peptides containing the four N-linked sites (N235, N340, N408 and N625) are displayed with the N-linked consensus sites underlined. The amino- and carboxyl-terminal residues of the tryptic peptides have been annotated with the respective amino acid numbers from the EphA4 Fc sequence. Any additional observed proteolytic cleavage by trypsin or Glu-C within the tryptic peptide sequences have also been annotated with the respective amino acid numbers. (B) Qualitative distribution of the glycan compositions observed from EphA4 Fc at N-linked sites N235, N340, N408 and N625.
