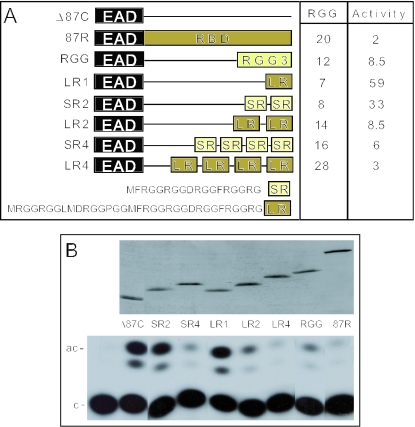
Figure 2.
An assay for repression by small regions of the EWS RBD. (A) Structure of experimental proteins. For reference, the structure of EWS, ATF1 and EWS/ATF1 are described in Figure 1. Δ87C (45) is a derivative of the EWS/ATF1 oncogene containing only the N-terminal 86 residues of the EAD fused to ATF1 (residues 66–271) including the bZIP domain that allows dimerization and DNA binding. Δ87C is a constitutive activator of ATF-dependent promoters dependent on the EAD region (45). RGG3 corresponds to residues 545–656 of intact EWS. All of the novel proteins tested are derived from Δ87C by insertion of the indicated sequence between the EAD and ATF1 regions. The ATF1 region is thus present in all proteins but is not shown in the diagram. The amino acid sequence of a short repeat (SR, light green box) and a long repeat (LR, dark green box) are present within the RGG3 region of EWS (LR contains EWS residues 571–604 and SR contains EWS residues 587–604). The number of RGG-tripeptides in each protein and the relative transcription activity are shown to the right. (B) Transcription assays. JEG3 cells were transfected with 5 μg of ATF-dependent reporter [pΔ−(71)SomCat] and 5 μg of plasmid expressing the activator indicated. Transcriptional activity was monitored by CAT assay and a representative TLC result is shown at the bottom (c = chloramphenicol; and ac = acetylated chloramphenicol). The corresponding western blot, using monoclonal antibody KT3 (45) to monitor epitope-tagged activator levels, is shown above the CAT assay.