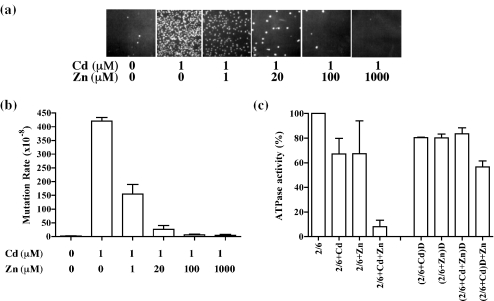
Figure 4.
Effect of zinc on the cadmium-induced inhibition of MMR. (a) Yeast mutator assay using a strain carrying the lys2-10A allele was preformed in the presence of 1 μM Cd2+ and 0–1000 μM Zn2+. The appearance of Lys+ revertant colonies indicates a mutator phenotype. (b) Effect of Zn2+ on the mutator rate of the lys2-10A strain in the presence of Cd2+. Each bar corresponds to the average of three sets of experiments using five independent colonies per set. Rates are calculated as described in Materials and Methods and standard deviation is included at the top of each bar. (c) MSH2–MSH6 (40 nM) was pre-incubated with 50 μM Cd2+ (2/6+Cd), 50 μM Zn2+ (2/6+Zn) or a combination of both (2/6+Cd+Zn) at 4°C for 10 min and assayed for ATPase activity as described in Materials and Methods. To remove excess metal ions, the mixture was dialyzed (denoted by a D in the Figure) extensively and assayed for ATPase activity. (2/6+Cd)D indicates that MSH2–MSH6 was treated with Cd2+ followed by dialysis; (2/6+Zn)D indicates that MSH2–MSH6 was treated with Zn2+ followed by dialysis; (2/6+Cd+Zn)D indicates that MSH2–MSH6 was treated with Cd2+ and Zn2+ followed by dialysis; and (2/6+Cd)D+Zn indicates that MSH2–MSH6 was treated with Cd2+ followed by dialysis, and then treated with Zn2+. A comparison of the ATPase activity of the dialyzed and undialyzed MSH2–MSH6 is shown. The activity of untreated, undialyzed MSH2–MSH6 was used as 100% and corresponds to 26 pmol ATP hydrolyzed. Each set of experiments was repeated three times and the average is presented together with the standard deviation.