Abstract
Osteosarcoma (OS) is the most common type of malignant bone tumor in children and adolescents. In total, 40–50% of patients with OS experience metastasis, and thus have a poor prognosis. Our previous study demonstrated that arsenic trioxide (As2O3) combined with doxorubicin [also known as Adriamycin (ADM)] significantly inhibited OS cell proliferation by downregulating stathmin expression. The present study investigated the effect and mechanism of stathmin expression on OS cell invasion. It was identified that the expression of stathmin was increased in human ADM-resistant OS MG63 (MG63/dox) cells compared with the level in the normal osteoblast hFoB1.19cell line using western blot analysis. Lentiviral-mediated small hairpin RNA (shRNA) was constructed to silence stathmin expression of MG63/dox cells. In transwell assay, stathmin-knockdown significantly suppressed migration and invasion in MG63/dox cells. As2O3 combined with ADM inhibited the migration and invasion of MG63/dox cells, and was associated with the downregulation of phosphorylated-mitogen-activated protein kinase (MAPK) 1 and β-catenin, and upregulation of phosphorylated-MAPK8 and E-cadherin. In addition, stathmin-knockdown significantly suppressed tumor growth and increased E-cadherin expression in a xenograft nude mouse model. Taken together, these data suggested that As2O3 combined with ADM inhibited stathmin-mediated invasion via the MAPK pathway. Elucidation of the mechanism for stathmin downregulation by As2O3 may provide novel insights into the mechanism of OS metastasis.
Keywords: osteosarcoma metastasis, arsenic trioxide, stathmin, E-cadherin, MAPK pathway
Introduction
Osteosarcoma (OS) is the most common type of primary malignant bone tumor in children and adolescents. OS accounts for almost 60% of malignant bone tumors in individuals <20 years old (1). Although the diagnosis and treatment of OS with adjuvant chemotherapy and local excision is improving, pulmonary metastasis occurs in 40–50% of patients with OS (2). When metastatic disease develops after radiotherapy with curative intent, the prognosis is particularly poor (3). Tumor metastasis remains a critical obstacle to the treatment of OS. Arsenic trioxide (As2O3) has been effective at inducing the apoptosis of certain types of cancer cells and solid tumors (4,5). We previously reported that As2O3 suppressed the growth of the human OS MG63 cell line, and reversed the doxorubicin (ADM) resistance of ADM-resistant MG63 (MG63/dox) cells by inducing apoptosis and downregulating stathmin expression in vitro (6). However, the effects of As2O3 on OS cell invasion and metastasis have yet to be clarified. Thus, the present study aimed to elucidate the role of As2O3 and stathmin in regulating the invasive activity of OS cells, in vitro and in vivo.
Stathmin (also known as oncoprotein 18) is a microtubule-destabilizing protein. Microtubules serve a critical role in the mediation of cell motility, which is inhibited if microtubules are stabilized. Stathmin is involved in tumor cell proliferation, invasion and motility (7).
The mitogen-activated protein kinase (MAPK) pathway is associated with a series of signaling cascades, mediating diverse intracellular responses. One potential target of MAPKs is stathmin, a protein that serves an important role in the regulation of microtubules, which are required for cell motility (8). In the present study, the MAPK pathway was demonstrated to be associated with the cellular response to As2O3. E-cadherin has been associated with metastatic progression in several types of cancer. The dissociation of the E-cadherin/β-catenin adhesion complex represents a key step in the epithelial-mesenchymal transition (EMT), a major mechanism for tumor cell metastasis. Repressing the expression of E-cadherin may promote EMT and induce cancer invasion (9,10). Thus, the effect of As2O3 on E-cadherin expression, and the association between stathmin and cell invasion, were also investigated in the present study.
Materials and methods
Cell lines
Human embryonic kidney HEK293T cells, the human OS MG63 cell line and the normal human osteoblast hFoB1.19cell line were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China). The human OSMG63/dox multidrug-resistant (MDR) cell line was provided by Dr Yoshio Oda (Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan). Cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare, Logan, UT, USA) and supplemented with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare), 100 U/ml penicillin and 100 g/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified, 5% CO2 atmosphere.
Reagents and antibodies
As2O3 was obtained from Beijing SL Pharmaceutical Co., Ltd. (Beijing, China) and ADM from Pfizer, Inc. (New York, NY, USA). PD098095 (cat. no. 9900) and SP600125 (cat. no. 8177) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against MAPK 3/1 (ERK1/2, cat. no. 5013) and 8 (JNK; cat. no. 9252), phosphorylated-ERK1/2 (P-ERK; cat. no. 4370) and phosphorylated-JNK (P-JNK, cat. no. 9255) were purchased from Cell Signaling Technology, Inc. Antibodies against E-cadherin (cat. no. ab15148) and β-catenin (cat. no. ab22656) were purchased from Abcam (Cambridge, UK). The antibody against β-actin (cat. no. cw0096 m) was purchased from Beijing Co Win Bioscience Co., Ltd (Beijing, China).
Invasion assay
Invasion assays were performed using a Transwell system (Corning Inc., Corning, NY, USA). The polycarbonate membrane of the chamber was coated with 250 µg/ml Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). The bottom chambers were filled with DMEM containing 10% FBS, and serum-free DMEM was added to the top chambers. A total of 2×104 cells treated with 2 µM As2O3 and/or 200 ng/ml ADM per well were added into the top chamber, followed by 24 h of incubation at 37°C with 5% CO2. MG63/dox cells incubated without drugs acted as the control group. The cells attached to the upper side of the filter were removed with a cotton swab. The cells invaded from the Matrigel onto the lower side of the Transwell inserts were fixed with 100% methanol, stained with crystal violet and counted under a light microscope.
Wound healing assay
A total of 5×105 cells were seeded into 6-well plates and incubated at 37°C for 6 h. A linear wound was created in the cellular monolayer using a sterile pipette tip, and then washing occurred with DMEM to remove cellular debris and yield an acellular area in each well. The cells were incubated with for 48 h with2 µM As2O3, 200 ng/ml ADM, both or neither. Images were captured of the wound areas and the location in the well was noted in terms of the distance between cells.
Western blotting
Cells were harvested using Trypsin-EDTA solution (Beyotime Institute of Biotechnology, Jiangsu, China), washed with PBS twice (15,000 × g, 4°C, 5 min) and lysed with radioimmunoprecipitaiton lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) on ice. The proteins from the lysed cells were extracted with centrifugation (15,000 × g, 4°C, 10 min). The protein concentrations were determined using an Evolution 60S UV-Visible spectrophotometer (Thermo Fisher Scientific, Inc.). A sample of 30 µg from each protein extract was separated using 8–12% SDS-PAGE, followed by western blot analysis using the previously described antibodies (dilution of antibodies: ERK1/2, JNK and β-actin: 1:1,000, P-ERK and P-JNK: 1:2,000, E-cadherin and β-catenin: 1:500). The membranes were incubated with primary antibody overnight at 4°C and washed with PBST three times for 10 min each time. Next the membranes were incubated with secondary antibody (Beijing CoWin Bioscience Co., Ltd.) for 1 h at room temperature. The relative protein level in the groups was normalized to a β-actin loading control. The gels were captured by Image Lab 3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The mean gray values were quantified using Gel-Pro Analyzer 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Lentiviral-mediated small hairpin RNA (shRNA) silencing of stathmin
The stathmin-specific targeting sequence designed by Shanghai Bioneer Science & Technology Co., Ltd. (Shanghai, China) was 5′-GUGUUGGUCUUUCUAAUGU-3′. pGCSIL-GFPshSTMN with pHelper1.0 and pHelper2.0 plasmids applied by Genomeditech Co., Ltd (Shanghai, China) were co-transfected into HEK293T cells and cultured overnight, then replaced with DMEM medium. At 48 h after transfection, the supernatant containing the virus was collected by centrifugation for 20 min at 1,000 × g. 4×107 TU/ml lentivirus containing shRNA against STMN (Lv-shSTMN) and a negative control (NC) virus were transfected into 2×106 MG63/dox cells at a multiplicity of infection of 20. Cells were subcultured at a 1:5 dilution in DMEM containing 300 µg/ml Geneticin (cat. no. A1720, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Positive stable transfectants were selected and cultured for further study. The clone in which the Lv-shSTMN vector was transfected was designated as the Lv-shSTMN group, and the NC vector-transfected clone was designated as the NC group.
Xenograft nude mouse model
Female, 6-week-old, immunodeficient, nude mice (BALB/c) were bred in the laboratory animal facility of the Children's Hospital of Suzhou University, housed individually in micro-isolator ventilated cages with free access to water and food. A total of 18 nude mice were randomly assigned to three groups: Transfected (Lv-shSTMN) or NC vector-transfected cells (5×106) suspended in 100 µl PBS were injected into the bone marrow cavity of the left tibia of each mouse. An equal number of age-matched animals received PBS in a volume of 100 µl were included as controls. The resulting tumor growths were measured with Vernier calipers on the 5, 8 and 12th and 16th day after xenograft formation. Tumor volume was calculated according to the following formula: Volume (mm3)=a2xb/2, where a and b represent the shortest and longest diameters, respectively. At the end of the experiment, the mice were sacrificed, and the tumors were removed and examined for E-cadherin expression.
Immunohistochemical staining of E-cadherin
The nude mouse tissue samples were fixed in 10% formalin, embedded in paraffin and sectioned into 4-µm thick slices. Following heat-induced antigen retrieval, immunohistochemical staining was performed using a rabbit monoclonal antibody against E-cadherin (dilution: 1:30; cat. no. ab15148). All procedures were performed according to the antibody manufacturer's protocol.
Statistical analysis
Statistical analysis was performed with SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation of three independent experiments. The statistical significance between groups was analyzed using the Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of stathmin in human OS cell lines
The expression of stathmin in human OSMG63 and MG63/dox, and human osteoblast hFoB1.19 cell lines was investigated. Western blotting results revealed that compared with the hFoB1.19 and MG63 cells, the MG63/dox cells exhibited a higher expression of stathmin; MG63/dox was therefore selected for the subsequent experiments (P<0.05; Fig. 1).
Figure 1.
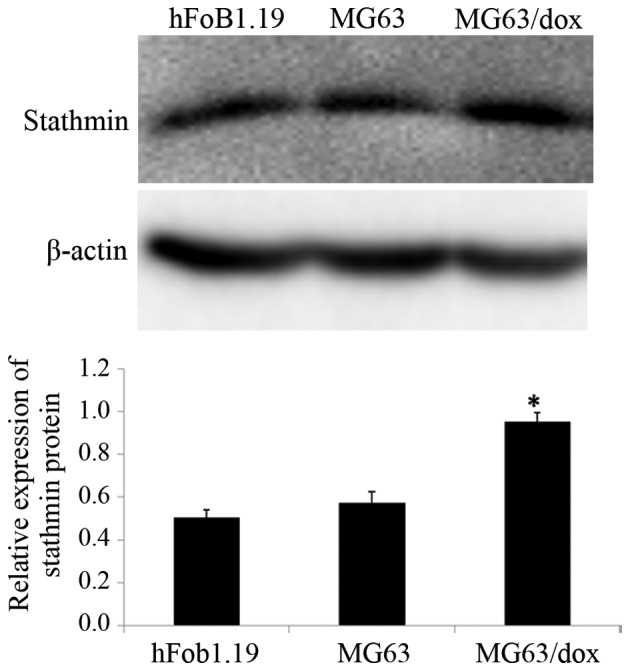
Quantification of the expression level of stathmin in human osteosarcoma cells (MG63 and MG63/dox) and osteoblast cells (hFoB1.19), compared with β-actin. Western blotting was employed to detect the expression of stathmin protein. The data are expressed as the mean ± standard deviation. *P<0.05 compared with hFoB1.19. MG63/dox, MG63/doxorubicin-resistant.
Effect of stathmin suppression on cell invasion
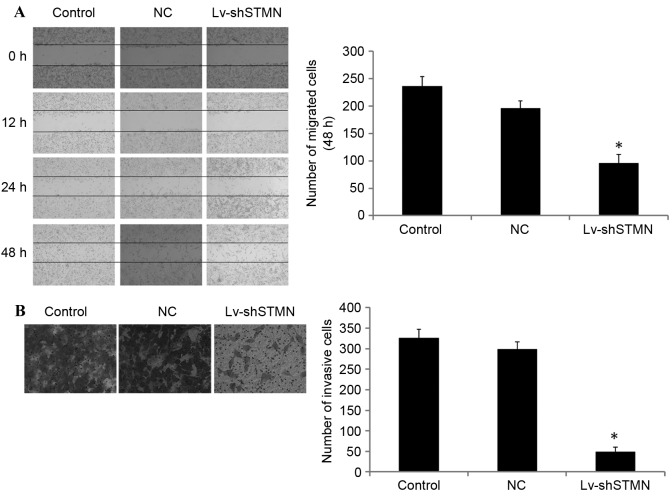
Lv-shSTMN was transfected into the MG63/dox cells to suppress the expression of stathmin (Fig. 2). The results indicated that the migration and invasion abilities were significantly reduced by stathmin suppression in the MG63/dox cells (P<0.05; Fig. 3).
Figure 2.
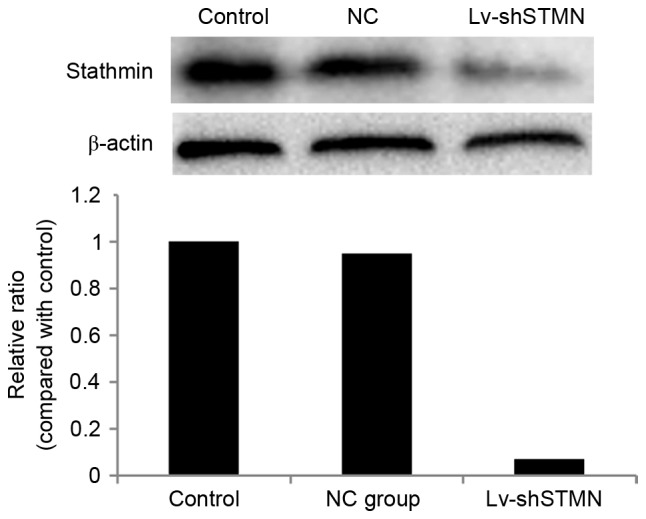
Effect of Lv-shSTMN transfection on the expression of stathmin. Lv-shSTMN and NC were transfected into cells. Positive stable transfectants were selected, and the expression level of stathmin protein was quantified with western blotting. The stathmin immunoreactive bands were normalized to β-actin bands. Lv-shSTMN, lentivirus with small hairpin RNA against stathmin; NC, negative control virus.
Figure 3.
Effect of stathmin inhibition on the migration and invasion of MG63/dox osteosarcoma cells. (A) Representative images of wound healing assays conducted on Lv-shSTMN- or NC-transfected MG63/dox cells. Images were captured at 0, 12, 24 and 48 h after scratching. (B) Representative images of a Transwell invasion assay conducted on the Lv-shSTMN- or NC-transfected MG63/dox. Images were captured after24 h. *P<0.05 compared with control group. MG63/dox, MG63/doxorubicin-resistant; Lv-shSTMN, lentivirus with small hairpin RNA against stathmin; NC, negative control virus.
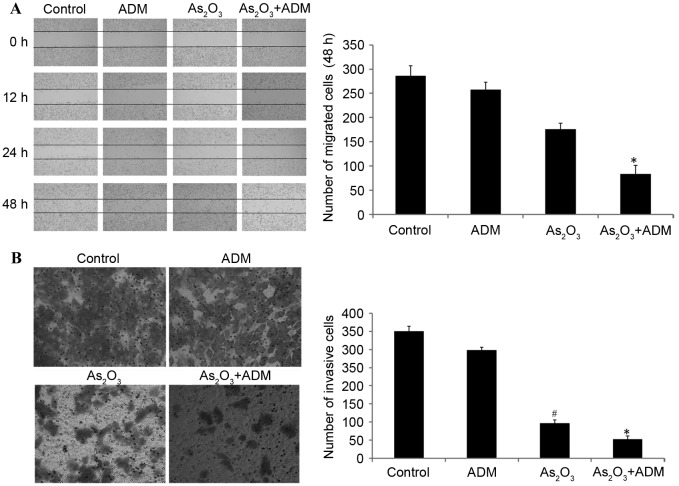
We previously reported that administration of As2O3 with ADM suppressed stathmin expression (6). The wound healing assay additionally demonstrated that the administration of As2O3 with ADM significantly inhibited cell migration compared with the control group (P<0.05; Fig. 4A).
Figure 4.
Effect of As2O3 and ADM on the migration and invasion of MG63/dox osteosarcoma cells. The cells were treated with 2 µM As2O3 and/or 200 ng/ml ADM for 48 h. (A) Images of the wound healing assay were captured at 0, 12, 24 and 48 h after scratching. (B) Images of the Transwell assay were captured after 24 h. #P<0.05, *P<0.05 compared with control group. As2O3, Arsenic trioxide; ADM, doxorubicin; MG63/dox, MG63/doxorubicin resistant.
Transwell invasion assays were conducted to investigate the effect of stathmin suppression on cell invasion. The result demonstrated that As2O3 alone or with ADM markedly inhibited tumor invasion potential compared with the control group (P<0.05; Fig. 4B).
Effect of combined As2O3 and ADM treatment on E-cadherin and β-catenin expression levels
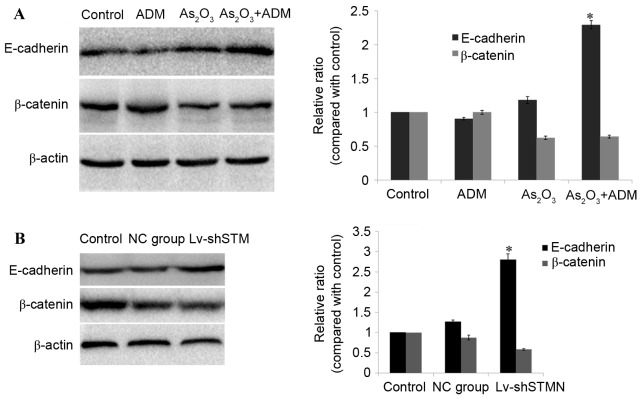
To investigate the molecular basis for As2O3 efficacy, MG63/dox cells were treated with As2O3, and the expression levels of E-cadherin and β-catenin were analyzed by western blotting. The results revealed that incubation with As2O3 and ADM simultaneously resulted in a significant upregulation of E-cadherin (P<0.05), and downregulation of β-catenin in MG63/dox cells (P=0.06; Fig. 5A).
Figure 5.
Effect of As2O3 and ADM combination treatment on E-cadherin and β-catenin expression levels. (A) The cells were treated with 2 µM As2O3 and/or 200 ng/ml ADM for 48 h. A western blot analysis was used to quantify the expression of E-cadherin and β-catenin. (B) Lv-shSTMN and NC were transfected into the cells. A western blot analysis was used to detect the expression of E-cadherin and β-catenin. The immunoreactive bands of E-cadherin and β-catenin protein were normalized to β-actin.*P<0.05 compared with control group. As2O3, arsenic trioxide; ADM, doxorubicin; Lv-shSTMN, lentivirus with small hairpin RNA against stathmin; NC, negative control virus.
The effect of shRNA-induced stathmin suppression on E-cadherin expression was also detected in MG63/dox cells. As presented in Fig. 5B, the expression level of E-cadherin was significantly increased (P<0.05) and the expression of β-catenin was reduced, but not significantly (P=0.08; Fig. 5B).
Effect of As2O3 and ADM combination treatment on JNK and ERK1/2 expression levels
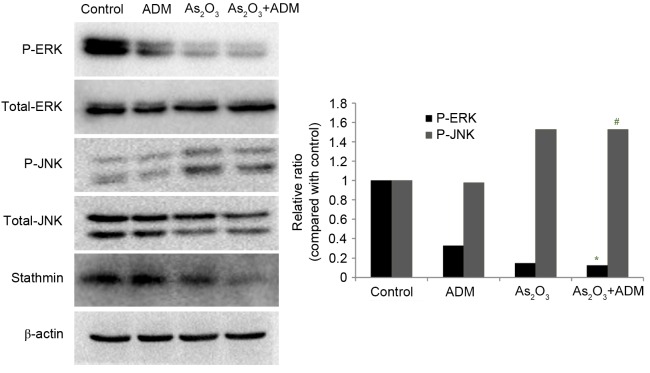
In order to evaluate the effect of As2O3 and ADM on the MAPK pathway, MG63/dox cells were incubated with As2O3 and ADM, and the expression levels of P-ERK and P-JNK were determined by western blot analysis, normalized to total ERK1/2 and JNK. The relative abundance of P-ERK was markedly reduced (P=0.015) whereas P-JNK expression was significantly increased (P=0.032) (Fig. 6).
Figure 6.
Effect of As2O3 and ADM combination treatment on JNK and ERK1/2 expression levels. MG63/dox cells were treated with 2 µM As2O3 and 200 ng/ml ADM for 48 h. A western blot analysis was used to detect the expression of ERK and JNK. The immunoreactive bands of the protein were normalized to β-actin bands. #P<0.05, *P<0.05 compared with control group. As2O3, arsenic trioxide; ADM, doxorubicin; JNK, mitogen-activated protein kinase 8; ERK1/2, mitogen-activated protein kinase 3/1; MG63/dox, MG63/doxorubicin-resistant.
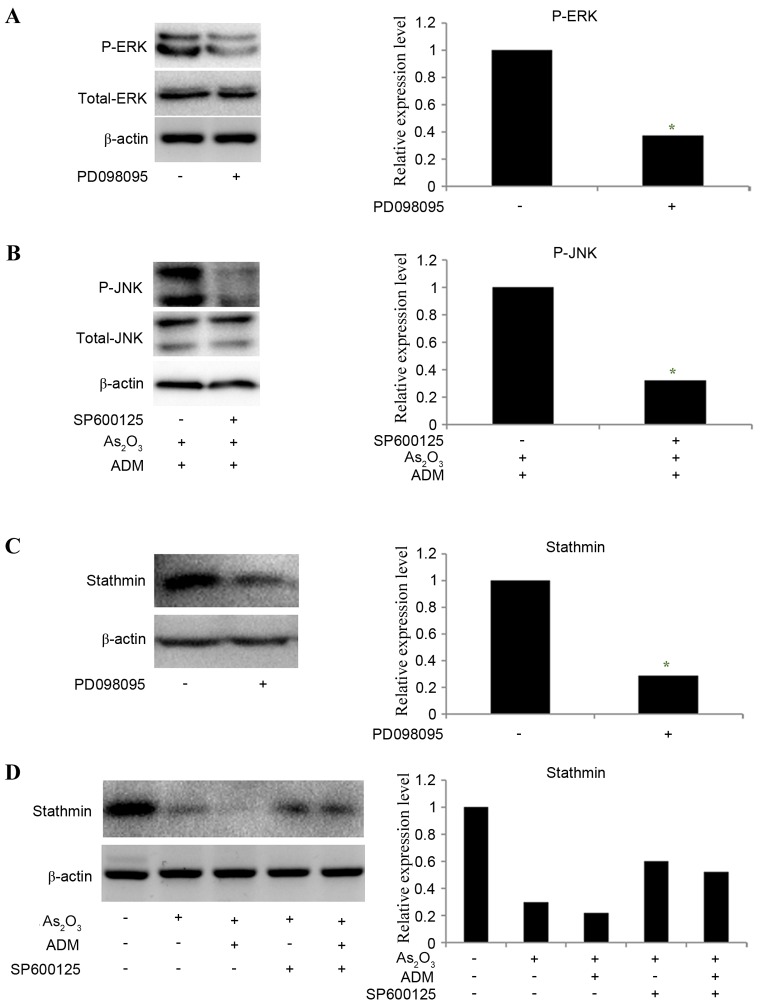
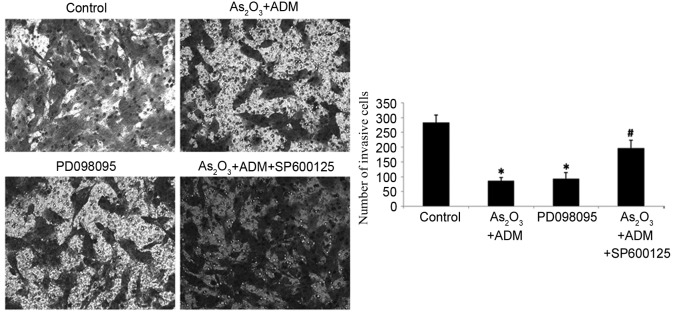
To determine whether ERK and JNK mediated the effect of As2O3 on the expression of stathmin, PD098095 and SP600125 were used to inhibit ERK and JNK expression, respectively. MG63/dox cells were incubated with As2O3 and ADM first to stimulate the JNK phosphorylation and then treated with SP600125. The results revealed that the relative levels of P-ERK (Fig. 7A) and P-JNK (Fig. 7B) were significantly decreased following inhibitor treatment (P-ERK, P=0.035; P-JNK P=0.025). The expression of stathmin was decreased following treatment withPD098095 (P=0.02; Fig. 7C). SP600125 partially reversed the effect of As2O3 and ADM on stathmin expression level (Fig. 7D). With the decline in stathmin expression, PD098095 also inhibited cell invasion, as determined by a Transwell assay, while SP600125 increased the number of invading cells following treatment with As2O3 and ADM (P<0.05; Fig. 8).
Figure 7.
Effect of ERK1/2 and JNK inhibitors on the level of P-ERK, P-JNK and stathmin. (A) A western blot analysis was used to detect the expression of ERK in MG63/dox cells treated with PD098095. (B) MG63/dox cells were treated with 2 µM As2O3 and 200 ng/ml ADM for 48 h. A western blot analysis was used to detect the expression of JNK in the cells treated with SP600125. The expression of stathmin in the MG63/dox cells with (C) PD098095 and (D) As2O3, ADM and SP600125 treatment was also detected by a western blot analysis. β-actin was used as a loading control. *P<0.05 compared with cells untreated with inhibitor. ERK1/2, mitogen-activated protein kinase 3/1; JNK, mitogen-activated protein kinase 8; P-ERK, phosphorylated-mitogen-activated protein kinase 3/1; P-JNK, phosphorylated-mitogen-activated protein kinase 8; MG63/dox, MG63/doxorubicin-resistant; As2O3, arsenic trioxide; ADM, doxorubicin.
Figure 8.
Effect of ERK1/2 and JNK inhibitors on the invasiveness of MG63/dox cells. A Transwell assay was used to assess the invasion ability of MG63/dox cells treated with As2O3 and ADM, PD098095, or As2O3 and ADM with SP600125. *P<0.05 compared with control group;#P<0.05 compared with the As2O3 and ADM group. ERK1/2, mitogen-activated protein kinase 3/1; JNK, mitogen-activated protein kinase 8; MG63/dox, MG63/doxorubicin-resistant; As2O3, arsenic trioxide; ADM, doxorubicin.
Effect of stathmin-knockdown on xenograft tumor growth
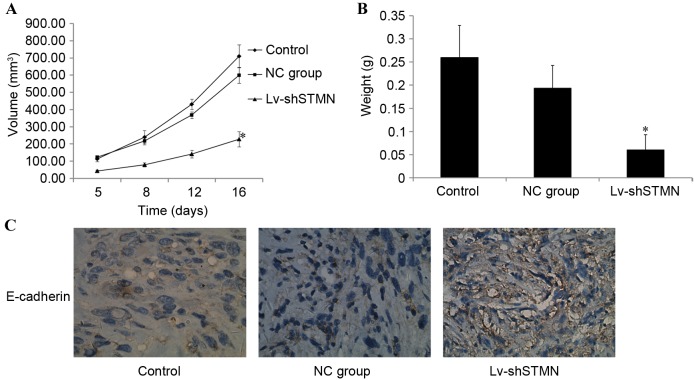
The in vitro experiments demonstrated the inhibitory effect of stathmin-knockdown on OS cell invasion and migration. The effect of stathmin on MG63/dox xenograft tumor growth in vivo was then investigated. Tumors that developed from Lv-shSTMN-transfected cells grew more slowly when compared with the control and NC groups (P<0.05; Fig. 9A). When the tumors were harvested, the mean weight of the tumors in the Lv-shSTMN group was significantly less than that in the NC and control groups (P<0.05; Fig. 9B). Immunohistochemistry analysis revealed that E-cadherin expression was markedly increased in the Lv-shSTMN group (Fig. 9C).
Figure 9.
Effect of Lv-shSTMN on MG63/dox xenograft tumor growth. A total of 5×106 stable Lv-shSTMN- or NC-transfected MG63/dox cells in 100 µl PBS were injected into the bone marrow cavity of the left tibia of an immunodeficient mouse model. (A) Tumor volumes were measured and growth curves for tumor proliferation were drawn accordingly. (B) The mice were sacrificed, and the weights of the tumors were measured. (C) Immunohistochemistry analysis revealed the level of E-cadherin expression in the xenograft. *P<0.05 compared with a non-transfected control group. Lv-shSTMN, lentivirus with small hairpin RNA against stathmin; NC, negative control virus; MG63/dox, MG63/doxorubicin-resistant.
Discussion
Cancer metastasis is a complex process that involves several molecular mechanisms. In the present study, the expression levels of stathmin in human OS and osteoblast cell lines were examined. Stathmin expression was increased in the OS cells compared with the normal cells. MG63/dox cells, which exhibit high stathmin expression, were selected and transfected with Lv-shSTMN to suppress stathmin. The results of the wound healing and Transwell invasion assays demonstrated that stathmin suppression inhibited cell migration and invasion. In order to further elucidate the function of stathmin in OS cell invasion, we intend to knock down stathmin expression in MG63 cells and construct stathmin overexpressing OS cells in the future.
Types of cancer with stathmin overexpression have been demonstrated to exhibit a more aggressive phenotype and a worse prognosis. Baldassarre et al (11) previously observed that in sarcomas, stathmin expression was higher in metastases than in primary tumors. Stathmin downregulation in sarcoma-derived cell lines inhibited migration, whereas overexpression increased FN-directed motility. Li et al (12) illustrated that stathmin expression promoted EMT through the regulation of microtubule dynamics, whereas Siva1 apoptosis inducing factor impeded cell migration and EMT by inhibiting stathmin. Liu et al (13) demonstrated that the knockdown of stathmin with small interfering RNA inhibited cell migration in esophageal squamous cell carcinoma. These results encouraged the present investigation of the effect of stathmin on the migration and invasion of MG63/dox cells. Stathmin expression was knocked down in MG63/dox cells using shRNA. A Transwell invasion assay revealed that stathmin suppression reduced the number of invasive cells. In our previous study, we demonstrated that treatment with As2O3 and ADM inhibited OS cell proliferation by downregulating stathmin expression (5). The wound healing and Transwell assays from the present study additionally demonstrated that As2O3 combined with ADM significantly inhibited cell migration and invasion via the inhibition of stathmin.
As stathmin interacts with proteins from various signaling pathways, the effect of the MAPK pathway on stathmin function was investigated. MAPKs are serine/threonine kinase proteins that have been identified in all eukaryotes. The MAPKs regulate a variety of oncogenic phenotypes, including proliferation, invasion, angiogenesis and inflammatory response (8). Chandhanayingyong et al (14) demonstrated the clinical benefit of MAPK/ERK-targeted therapy for patients with unresectable or metastatic OS. Miao et al (15) reported that the knockdown of endogenous galanin expression in MG63 cells reduced proliferation and invasion of the tumor cells via the suppression of MAPK/ERK expression. The present study revealed that treatment with As2O3 and ADM increased the phosphorylation of JNK and decreased ERK1/2 phosphorylation, which may suggest that As2O3 and ADM effect stathmin expression through the MAPK pathway. In order to verify this result, PD098095 and SP600125 were used to inhibit ERK and JNK, respectively. It was revealed that PD098095 decreased stathmin expression and the number of invasive cells. SP600125 partially reversed the effect of As2O3 on stathmin expression. This indicated that As2O3 inhibited stathmin mediated-invasion via the ERK/JNK MAPK signaling pathways. In accordance with the in vitro results, knockdown of stathmin inhibited the growth of OS cell in vivo, suggesting that stathmin may present an effective target for antitumor therapy. The regulating mechanism of the ERK/JNKMAPK signal pathways on stathmin is to be investigated in greater detail in further studies.
E-cadherin/β-catenin signaling mediates the contact inhibition of normal epithelial cells. The abnormal expression of E-cadherin and β-catenin in cancer causes a decline in cell adhesion. Cancer cells escape the normal regulatory mechanism of contact inhibition to become more proliferative and metastasize (16,17). Thus, E-cadherin is an important tumor and metastasis suppressor gene. In the present study, the expression level of E-cadherin was quantified. The result revealed that As2O3 and ADM treatment increased E-cadherin expression, decreasing cell invasion potential accordingly. To simulate the growth conditions of OS, a model was produced with the in situ vaccination of mice. Although no attempt was made to detect metastatic foci in this model, immunohistochemistry demonstrated that the E-cadherin expression levels of the tumors in the stathmin group were higher than those in the control group. This suggested the metastatic ability of the tumors would be poor following stathmin-knockdown. The result was verified in vitro. E-cadherin is anchored to the cytoskeleton by catenin, to form stable connections with neighboring cells. The knockdown of stathmin in MG63/dox cells may have prompted β-catenin phosphorylation and proteasomal degradation. The E-cadherin/β-catenin complex then disaggregated, affecting the stability of cell junctions. The E-cadherin/β-catenin pathway may act downstream of stathmin to regulate migration and invasion (18). In a further study, a mouse model will be constructed by intravenous injection into the tail to allow the effect of stathmin on in vivo OS cell invasion to be investigated in detail.
In conclusion, the present study data demonstrated that stathmin function is associated with the migration and invasion, and therefore, the metastasis of OS cells. As2O3treatment decreased stathmin expression and may have inhibited the growth and invasion of OS cells via the ERK/JNKMAPK pathways. This observation provides an insight into the function of stathmin in cancer progression and indicates that a decrease in stathmin may contribute to the metastasis of OS. Stathmin is therefore a promising biomarker and therapeutic target for metastatic OS.
References
- 1.Man TK, Chintagumpala M, Visvanathan J, Shen J, Perlaky L, Hicks J, Johnson M, Davino N, Murray J, Helman L, et al. Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer Res. 2005;65:8142–8150. doi: 10.1158/0008-5472.CAN-05-0985. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Chalmers PN, Morris CD. Pediatric osteogenic sarcoma. Curr Opin Pediatr. 2010;22:61–66. doi: 10.1097/MOP.0b013e328334581f. [DOI] [PubMed] [Google Scholar]
- 3.Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z, Aina H, Yuanjue S, Daliu M, Zan S, Yang Y. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: A meta-analysis. Surg Oncol. 2012;21:e165–e170. doi: 10.1016/j.suronc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bachleitner-Hofmann T, Kees M, Gisslinger H. Arsenic trioxide: Acute promyelocytic leukemia and beyond. Leuk Lymphoma. 2002;43:1535–1540. doi: 10.1080/1042819021000002857. [DOI] [PubMed] [Google Scholar]
- 5.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 6.Feng T, Qiao G, Feng L, Qi W, Huang Y, Yao Y, Shen Z. Stathmin is key in reversion of doxorubicin resistance by arsenic trioxide in osteosarcoma cells. Mol Med Rep. 2014;10:2985–2992. doi: 10.3892/mmr.2014.2618. [DOI] [PubMed] [Google Scholar]
- 7.Niethammer P, Bastiaens P, Karsenti E. Stathmin-tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862–1866. doi: 10.1126/science.1094108. [DOI] [PubMed] [Google Scholar]
- 8.Hu JY, Chu ZG, Han J, Dang YM, Yan H, Zhang Q, Liang GP, Huang YS. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010;67:321–333. doi: 10.1007/s00018-009-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 10.Chen HN, Yuan K, Xie N, Wang K, Huang Z, Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, Huang C. PDLIM1 stabilizes the E-cadherin/β-catenin complex to prevent epithelial-mesenchymal transition and metastatic potential of colorectal cancer cells. Cancer Res. 2016;76:1122–1134. doi: 10.1158/0008-5472.CAN-15-1962. [DOI] [PubMed] [Google Scholar]
- 11.Baldassarre G, Belletti B, Nicoloso MS, Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V, Colombatti A. p27 (Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Jiang P, Du W, Wu Z, Li C, Qiao M, Yang X, Wu M. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules; Proc Natl Acad Sci USA; 2011; pp. 12851–12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Sun YL, Xu Y, Liu F, Wang LS, Zhao XH. Expression and phosphorylation of stathmin correlate with cell migration in esophageal squamous cell carcinoma. Oncol Rep. 2013;29:419–424. doi: 10.3892/or.2012.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandhanayingyong C, Kim Y, Staples JR, Hahn C, Lee FY. MAPK/ERK signaling in osteosarcomas, Ewing sarcomas and chondrosarcomas: Therapeutic implications and future directions. Sarcoma. 2012;2012:404810. doi: 10.1155/2012/404810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao JH, Wang SQ, Zhang MH, Yu FB, Zhang L, Yu ZX, Kuang Y. Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncol Rep. 2014;32:1497–1504. doi: 10.3892/or.2014.3358. [DOI] [PubMed] [Google Scholar]
- 16.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faux MC, Coates JL, Kershaw NJ, Layton MJ, Burgess AW. Independent interactions of phosphorylated β-catenin with E-cadherin at cell-cell contacts and APC at cell protrusions. PLoS One. 2010;5:e14127. doi: 10.1371/journal.pone.0014127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/β-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]