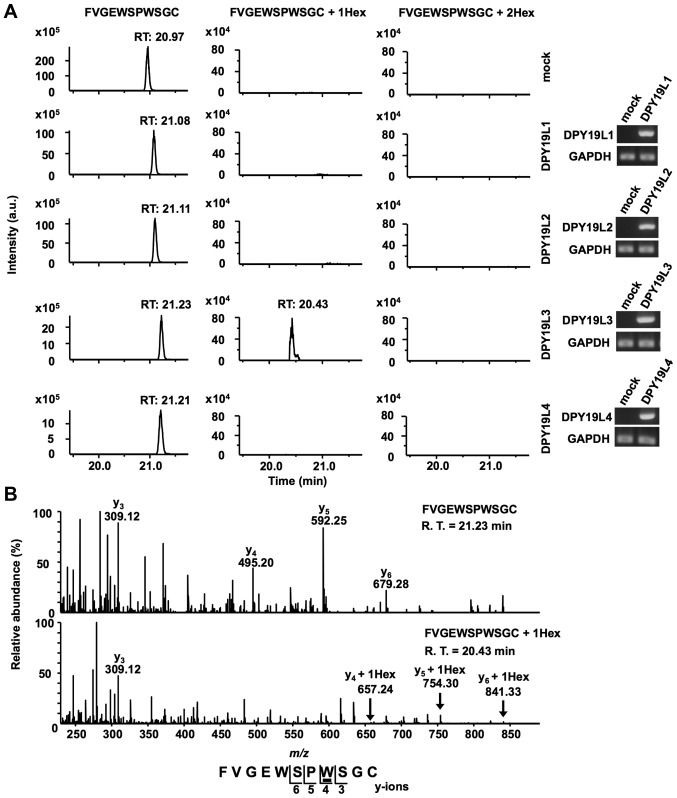
Figure 3.
DPY19L3 is the C-mannosyltransferase of RPESP at only W83. (A and B) Identification of C-mannosyltransferase of RPESP. Human DPY19L1-L4 or empty vector (mock) and pMT-RPESP-MH were transiently transfected into Drosophila S2 cells and protein expression was induced using treatment with 700 µM CuSO4 for 48 h. RPESP-MH protein was purified with Ni-NTA agarose. Following aminoethylation, the samples were digested with trypsin, and the resulting peptides were analyzed by targeted MS/MS method. According to the inclusion list of three doubly protonated parent ions of un-mannosylated (m/z, 649.28), mono-mannosylated (m/z, 730.31) and di-mannosylated 76FVGEWSPWSGC86 peptides (m/z, 811.34), MS/MS spectra were obtained. Selected ion chromatograms of y5 ion of these parent ions were determined (A, left panel). The expression of transfected DPY19L1-L4 mRNAs in S2 cells was confirmed by reverse transcription-semi-quantitative polymerase chain reaction (A, right panel). The MS/MS spectra of the doubly charged un- (B, upper panel) and mono- (B, lower panel) mannosylated peptide ion derived from DPY19L3-expressing S2 cells are presented. The indicated y-series ions were detected as singly charged ions and only the W83 residue of RPESP was C-mannosylated. C-mannosyltryptophan is underlined. DPY19L, dpy-19 like; MS, mass spectrometry; RPESP, RPE-spondin; Hex, hexose; R.T., retention time.