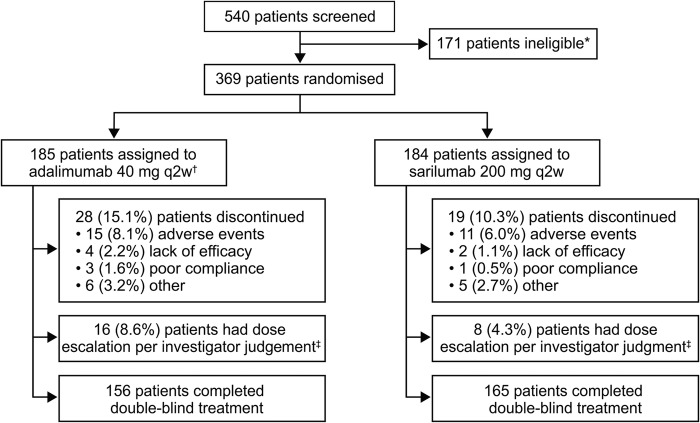
Figure 1.
Flow diagram showing patient disposition. *Primary reasons for patient ineligibility were meeting the exclusion criteria related to tuberculosis (12.0%) or failure to meet the inclusion criterion for severity of disease (8.1%). †One patient was randomised but not treated in the adalimumab group. ‡The actual number of patients who received a dose-escalation kit on the basis of meeting protocol criteria were 6 (3.2%) in the adalimumab group and 5 (2.7%) in the sarilumab group. q2w, every 2 weeks.