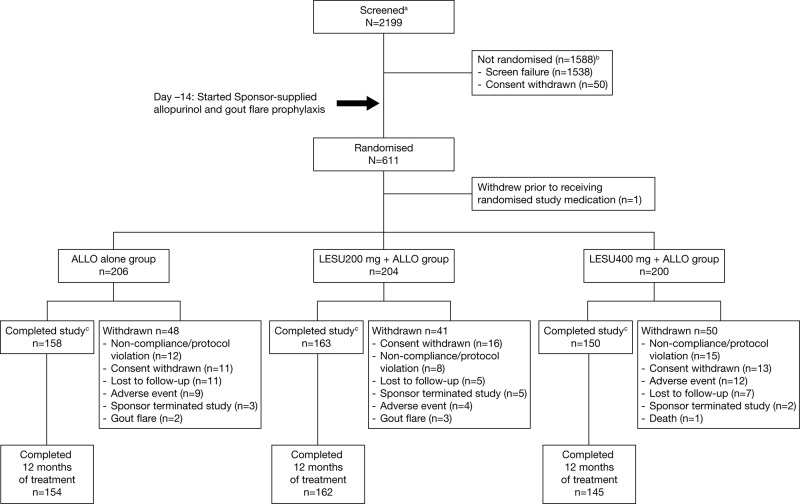
Figure 2.
Patient disposition is shown. aScreened was defined as signing an informed consent form; b2 deaths reported for non-randomised patients during screening and ccompleted the study with or without completing randomised study medication. One additional death occurred in the LESU 400 mg+ALLO group. The subject experienced a serious adverse event and withdrew from the study. The primary reason for study withdrawal was reported as ‘adverse event’. Of the 1538 screen failures, 1183 were related to inclusion criteria, 252 to exclusion criteria, 94 to both inclusion and exclusion criteria and 9 to other.
ALLO, allopurinol; LESU, lesinurad.