Table 2.
IRs (patients with events/100 patient-years; 95% CI) of AEs and SAEs (all-cause)
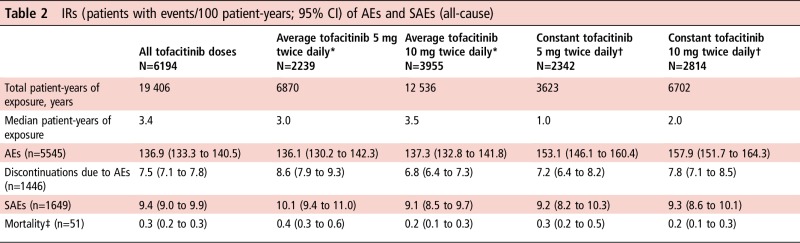
|
*Average dosing was based on average daily dose: patients receiving <15 mg/day were assigned to the 5 mg twice daily group; patients receiving ≥15 mg/day were assigned to the 10 mg twice daily group.
†Constant dosage without prior exposure to another tofacitinib dose or adalimumab during the study; patients who switched doses were not included in this group.
‡Within 30 days of last dose of study drug.
AE, adverse event; IR, incidence rate; n, unique number of patients with event; SAE, serious AE.
