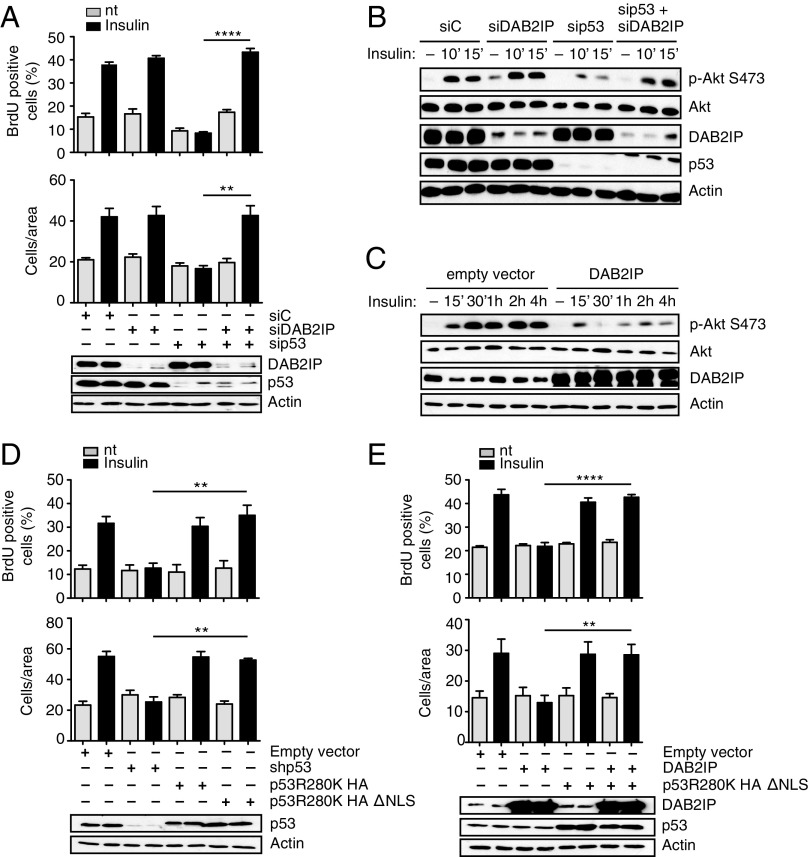
Fig. 3.
Functional interaction between mutant p53 and DAB2IP in the response of cancer cells to insulin. (A) Epistasis between mutp53 and DAB2IP in insulin-induced proliferation and invasion. MDA-MB231 cells were silenced for mutp53 and/or DAB2IP for 48 h and treated with 0.5 μg/mL insulin for 24 h. Proliferation (Top) and invasion (Bottom) assays were performed as in Fig. 1 (mean ± SEM; n = 3; **P < 0.01, ****P < 0.0001). nt, not treated. (B and C) Epistasis between mutant p53 and DAB2IP in insulin-induced AKT activation. (B) MDA-MB231 cells were silenced for mutp53 and/or DAB2IP for 48 h, serum-starved for 24 h, and treated with insulin (0.5 μg/mL) for the indicated times. Phosphorylated AKT1 and total AKT1 were detected by immunoblotting. (C) MDA-MB231 cells stably transduced with a retrovirus expressing DAB2IP were serum-starved and treated with insulin (0.5 μg/mL) for the indicated times. Phosphorylated AKT1 and total AKT1 were detected by immunoblotting. Total AKT is taken from a different gel, loaded in parallel with the same amount of lysate. (D and E) Insulin-induced proliferation and invasion are mediated by cytoplasmic mutant p53. (D) MDA-MB231 cells stably silenced for endogenous mutant p53 [short hairpin p53 (shp53)] were infected with retroviruses encoding shRNA-resistant versions of p53 (R280K) or its cytoplasmic variant p53 (R280K)ΔNLS. Proliferation (Top) and invasion (Bottom) assays were performed as in Fig. 1 (mean ± SEM; n = 3; **P < 0.01). (E) MDA-MB231 cells stably overexpressing DAB2IP were infected with a retrovirus expressing HA-p53 (R280K)ΔNLS or an empty retrovirus as a control. Proliferation (Top) and invasion (Bottom) assays were performed as in Fig. 1 (mean ± SEM; n = 3; **P < 0.01, ****P < 0.0001).