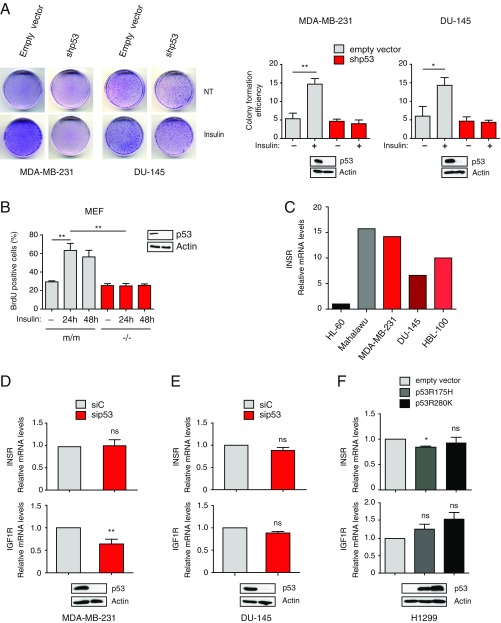
Fig. S1.
Mutant p53 depletion reduces insulin-induced cell proliferation without affecting expression of INSRs (related to Fig. 1). (A) Mutp53 sustains insulin-induced cell proliferation. MDA-MB231 and DU145 cells stably silenced for endogenous mutant p53 (shp53) were plated at low density and treated with 0.5 μg/mL insulin every 48 h for 10 d. Petri dishes were photographed after Giemsa staining (representative photographs are shown), and colony formation efficiency was quantified using ImageJ (mean ± SEM; n = 3; *P < 0.1, **P < 0.01). NT, not treated. (B) Mutp53 drives insulin-induced proliferation in Ras-transformed MEFs. MEFs derived from p53-null (−/−) or p53R172H knock-in (m/m) mice were treated and analyzed as in Fig. 1A (mean ± SEM; n = 3; **P < 0.01). Expression of p53 was checked by Western blot. (C) Expression of INSR in various human cancer cell lines. Expression of INSR A/B was measured by RT-quantitative PCR (RT-qPCR; n = 1) in the indicated cell lines. HL-60 and Malahawu cell lines were analyzed as negative (low INSR expression) and positive (high INSR expression) controls, respectively. Effects of mutant p53 depletion on expression of INSRs. MDA-MB-231 (D) and DU-145 (E) cells were transfected with the indicated siRNAs. Expression of INSR A/B and IGF1R was measured by RT-qPCR (mean ± SEM; n = 3; **P < 0.01). ns, statistically not significant at P > 0.05. Immunoblotting confirmed depletion of endogenous mutant p53. (F) Effects of mutant p53 overexpression on INSR and IGF1R mRNA levels. H1299 p53-null cells were infected with retroviruses expressing the indicated mutp53. Expression levels of INSR A/B and IGF1R were measured by RT-qPCR (mean ± SEM; n = 3; *P < 0.1). Immunoblotting confirmed expression of exogenous mutp53.