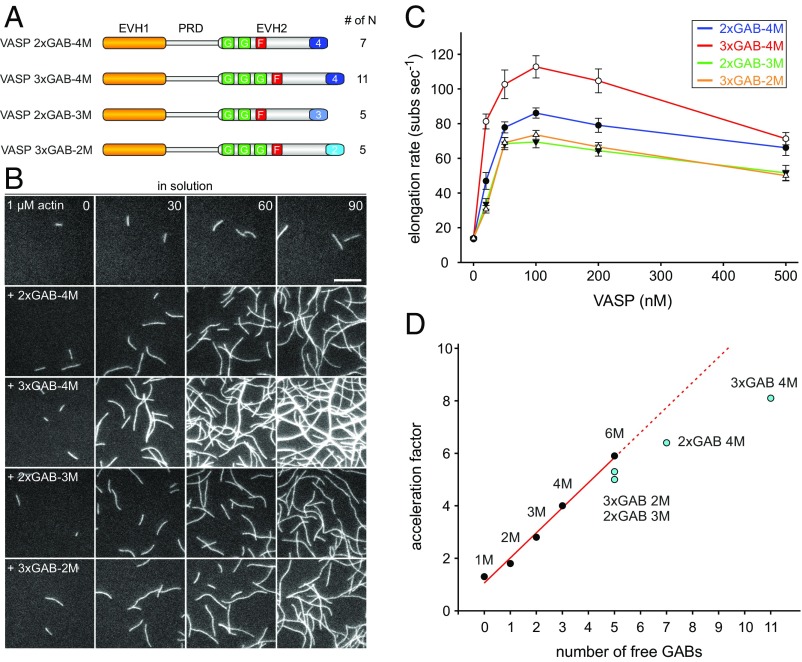
Fig. 2.
Additional GABs in the same polypeptide chain of VASP are less efficient to accelerate filament elongation in solution at higher N values. (A) Schematic domain organization of generated chimeric VASP mutants containing additional Dictyostelium GABs in the EVH2 domain. (B) Time-lapse micrographs of TIRFM assays for determination of elongation rates. The actin (1 μM, 23% Alexa 488 labeled) was polymerized in absence or presence of 200 nM of the indicated VASP construct in TIRF buffer, respectively. Time is given in seconds. (Scale bar, 5 µm.) (C) Comparison of the actin-filament elongation rates derived from TIRFM movies as shown in B in the presence of different VASP mutants at the concentrations indicated. Concentrations are given in monomers. Data correspond to means ± SD. Fifteen actin control filaments and 30 filaments for all other data points in presence of VASP were analyzed. (D) Comparison of the acceleration factors of VASP oligomerization construct and VASP constructs containing multiple GABs at 100 nM (relating to monomer concentration). Red line shows linear fit. Note less augmented filament elongation rates with VASP constructs containing multiple GABs within the same EVH2 domain.