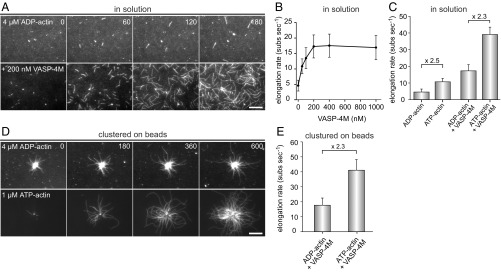
Fig. 4.
VASP does not require actin ATP hydrolysis of terminal subunits for filament elongation. (A) TIRF images of 4 µM ADP-actin (23% Alexa 488 labeled) polymerized in 1× ADP-TIRF buffer in the absence (Top) or presence (Lower) of 200 nM VASP-4M. Time is given in seconds. (B) Elongation rates of ADP-actin in the presence of increasing concentrations of VASP-4M in solution. (C) Comparison of elongation rates of ADP-actin or ATP-actin in absence or presence of 200 nM VASP-4M. (D) TIRFM images of 4 µM ADP-actin (Top) and 1.0 µM ATP-actin (Lower) polymerized in presence of VASP-4M–coated microspheres and 40 nM CP. (E) Comparison of elongation rates from ADP-G-actin and ATP-G-actin in the presence VASP-4M–derivatized beads. Note similar decrease in presence of ADP-actin for spontaneous and VASP-mediated actin assembly. (A and D) Time is given in seconds. (Scale bars, 20 µm.) (B, C, and E) Data correspond to means ± SD. Fifteen actin control filaments and 30 filaments for all other data points in presence of VASP-4M were analyzed.