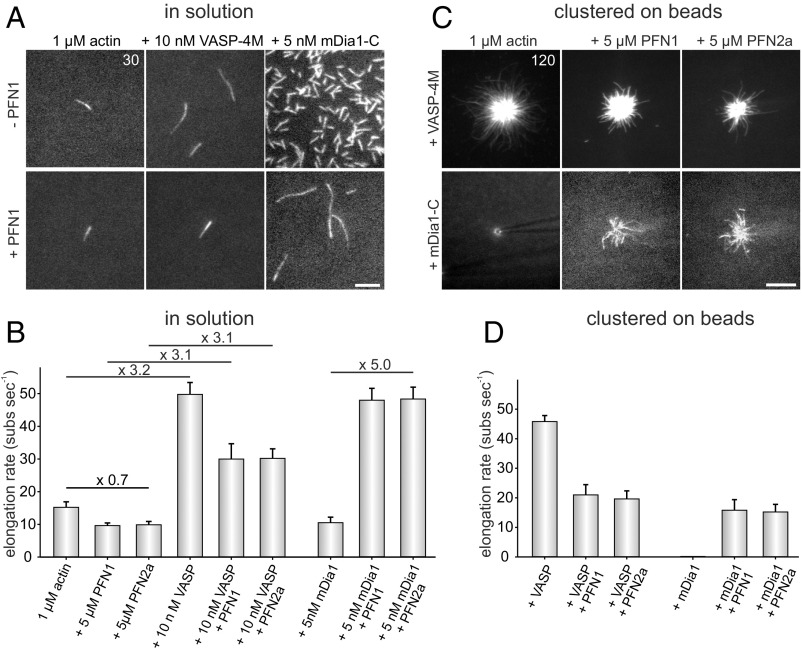
Fig. 5.
Profilin does not accelerate VASP-mediated filament elongation but is required to increase speed of formin-driven actin assembly. (A) Time-lapse micrographs of single TIRFM assays for determination of elongation rates in solution. The actin (1 μM, 23% Alexa 488 labeled) was polymerized in the absence or presence of either 5 µM PFN1, 10 nM VASP-4M, or 5 nM mDia1-C in TIRF buffer, respectively. Time is given in seconds. (Scale bar, 5 µm.) (B) Quantification of the elongation rates in solution derived from TIRFM experiments as shown in A. Bars represent means ± SD from at least three independent experiments. (C) Time-lapse micrographs of single TIRFM assays for determination of elongation rates in clustered arrays on beads. The actin (1 μM, 23% Alexa 488 labeled) was polymerized in absence or presence of either 5 µM PFN1 or PFN2a in TIRF buffer, respectively using SNAP-tag derivatized VASP-4M or mDia1-C beads. The spontaneous assembly of actin was inhibited by addition of 40 nM CP. No filament growth from mDia1-C–derivatized beads in the absence of PFN was observed. Time is given in seconds. (Scale bar, 10 µm.) (D) Quantification of the elongation rates on beads derived from TIRFM experiments as shown in C. Bars represent means ± SD from at least three independent experiments.