Abstract
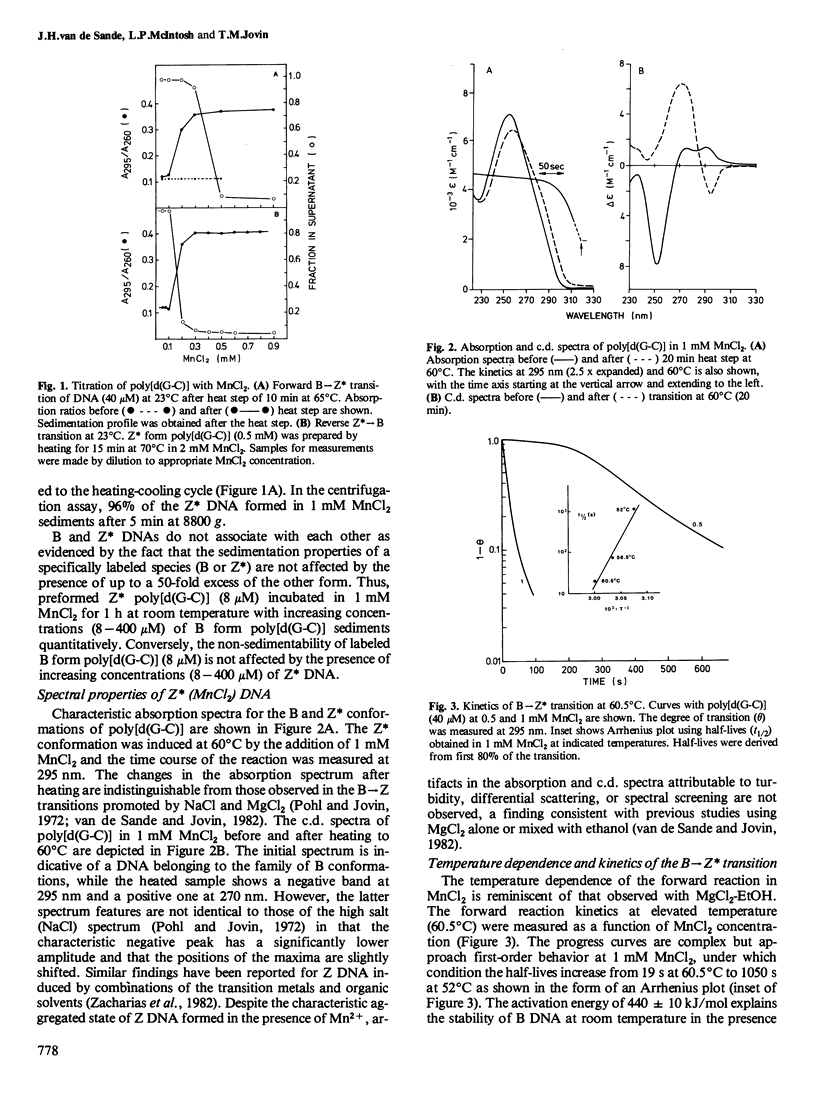
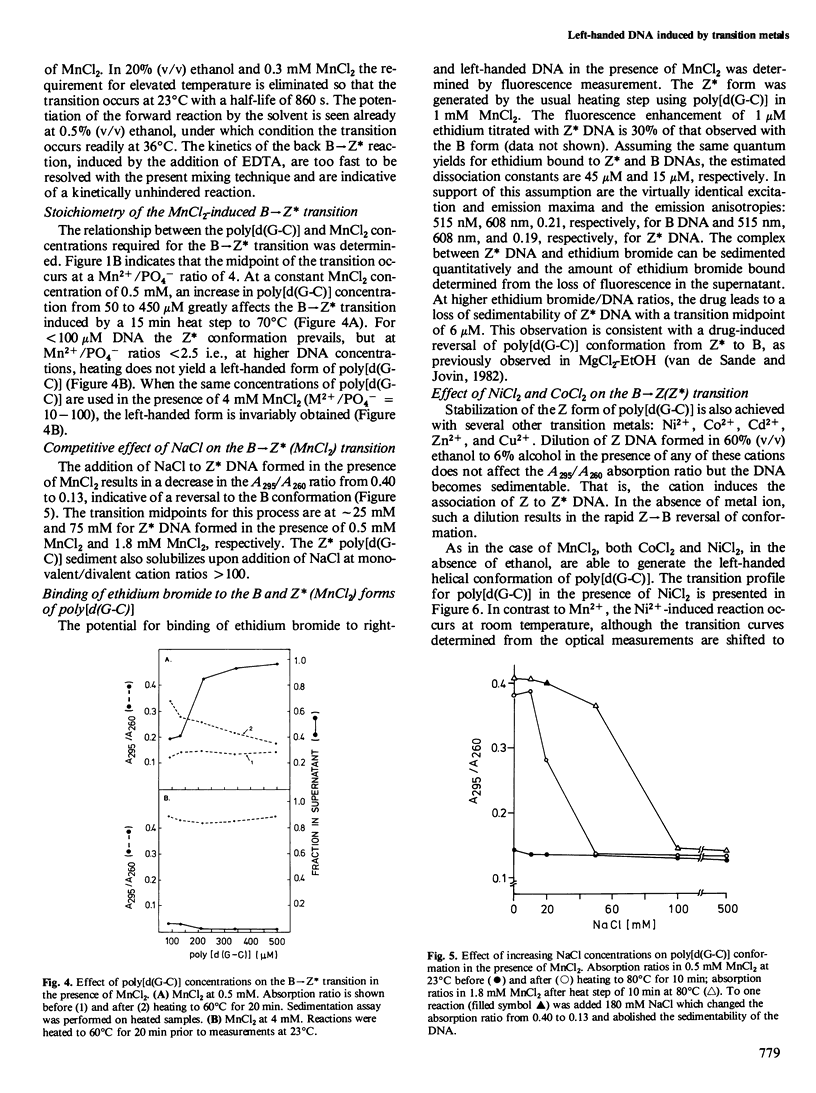
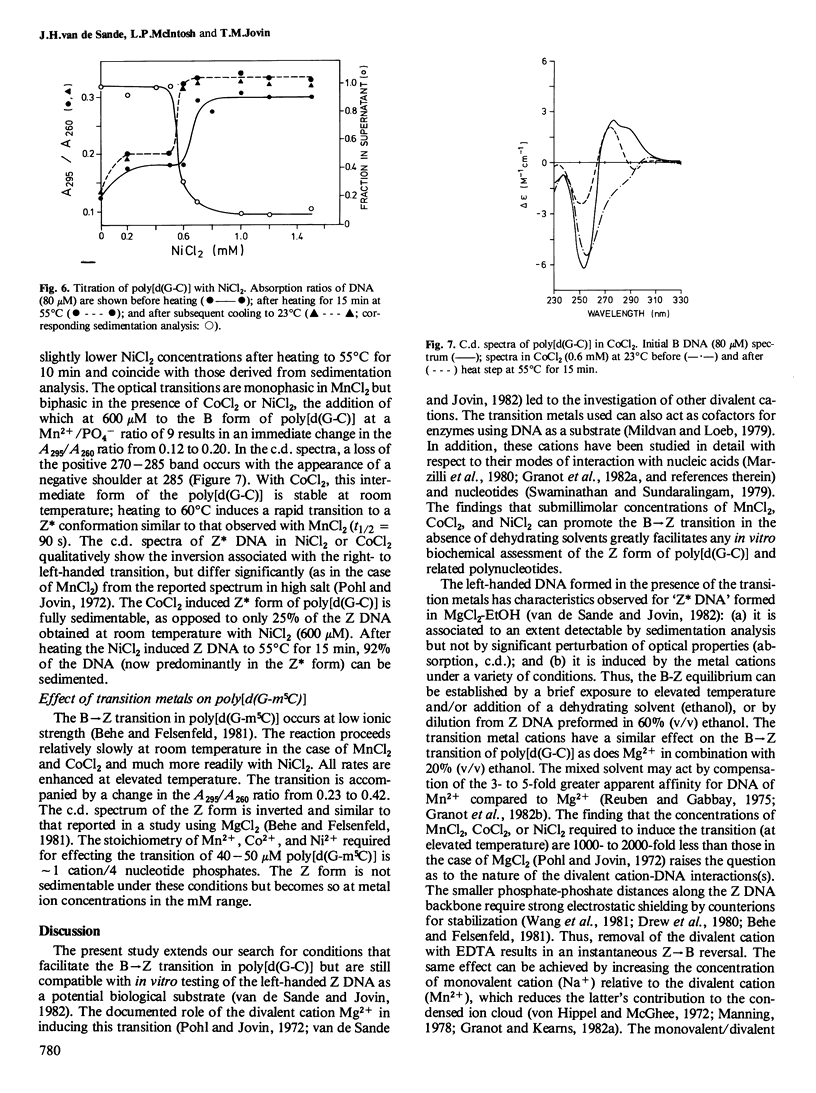
The effects of the first-row transition metal ions on the right(B)- to left(Z)-handed helical transition of poly[d(G-C)] have been determined. The Z conformation is induced by MnCl2 at submillimolar concentrations. The forward reaction has a very large activation energy (440 kJ/mol) so that a facile conversion occurs only at temperatures above 45 degrees C. However, the left-handed form remains stable upon cooling. The addition of ethanol (20% v/v) eliminates the requirement for elevated temperature. The transition is highly co-operative and is accompanied by spectral changes (absorption, circular dichroism) characteristic for the B----Z conformational transition. NiCl2 and CoCl2 also induce the B----Z transition in poly[d(G-C)] but the activation energies and thus the temperature requirements for the forward reaction are lower than those observed with MnCl2. The left-handed DNA formed in the presence of Mn2+ is similar to 'Z DNA' previously described in Mg2+-EtOH (van de Sande and Jovin , 1982): (a) it readily sediments out of solution at low speed as a consequence of intermolecular association which, however, is not accompanied by turbidity; and (b) it supports the binding of ethidium bromide although this drug interacts preferentially with the B form of DNA. With Ni2+, the B----Z isomerization step can be separated from the subsequent specific Z----Z* association. Mn2+, Ni2+, and Co2+ also promote the B----Z transition of poly[d(G-m5C)] at substoichiometric concentrations with respect to DNA nucleotide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Bond P. J., Chandrasekaran R. Visualization of an unwound DNA duplex. Nature. 1980 Oct 9;287(5782):561–563. doi: 10.1038/287561a0. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Zimmerman S., Felsenfeld G. Changes in the helical repeat of poly(dG-m5dC) . poly(dG-m5dC) and poly(dG-dC) . poly(dG-dC) associated with the B-Z transition. Nature. 1981 Sep 17;293(5829):233–235. doi: 10.1038/293233a0. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Eichhorn G. L. Interaction of metal ions with polynucleotides and related compounds. XV. Nuclear magnetic resonance studies of the binding of copper(II) to nucleotides and polynucleotides. Biochemistry. 1971 May 11;10(10):1857–1864. doi: 10.1021/bi00786a020. [DOI] [PubMed] [Google Scholar]
- Bregadze V. G. Interpretatsiia ul'trafioletovykh differentsial'nykh spektrov DNK v komplekse s nekotorymi ionami pervogo perekhodnogo riada. Biofizika. 1974 Jan-Feb;19(1):179–181. [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Conformation and dynamics in a Z-DNA tetramer. J Mol Biol. 1981 Nov 15;152(4):723–736. doi: 10.1016/0022-2836(81)90124-8. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Granot J., Assa-Munt N., Kearns D. R. Interactions of DNA with divalent metal ions. IV. Competitive studies of Mn2+ binding to AT- and GC-rich DNAs. Biopolymers. 1982 May;21(5):873–883. doi: 10.1002/bip.360210502. [DOI] [PubMed] [Google Scholar]
- Granot J., Feigon J., Kearns D. R. Interactions of DNA with divalent metal ions. I. 31P-NMR studies. Biopolymers. 1982 Jan;21(1):181–201. doi: 10.1002/bip.360210115. [DOI] [PubMed] [Google Scholar]
- Granot J., Kearns D. R. Interactions of DNA with divalent metal ions. II. Proton relaxation enhancement studies. Biopolymers. 1982 Jan;21(1):203–218. doi: 10.1002/bip.360210116. [DOI] [PubMed] [Google Scholar]
- Granot J., Kearns D. R. Interactions of DNA with divalent metal ions. III. Extent of metal binding: experiment and theory. Biopolymers. 1982 Jan;21(1):219–232. doi: 10.1002/bip.360210117. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Mizusawa H., Kakefuda T. Unwinding of double-stranded DNA helix by dehydration. Proc Natl Acad Sci U S A. 1981 May;78(5):2838–2842. doi: 10.1073/pnas.78.5.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck G., Zimmer C. Conformational aspects and reactivity of DNA. Effects of manganese and magnesium ions on interaction with DNA. Eur J Biochem. 1972 Sep 25;29(3):528–536. doi: 10.1111/j.1432-1033.1972.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Loeb L. A. The role of metal ions in the mechanisms of DNA and RNA polymerases. CRC Crit Rev Biochem. 1979;6(3):219–244. doi: 10.3109/10409237909102564. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Nichols S. R., Rich A. 7-Methylguanine in poly(dG-dC).poly(dG-dC) facilitates z-DNA formation. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4777–4781. doi: 10.1073/pnas.78.8.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Reuben J., Gabbay E. J. Binding of manganese(II) to DNA and the competitive effects of metal ions and organic cations. An electron paramagnetic resonance study. Biochemistry. 1975 Mar 25;14(6):1230–1235. doi: 10.1021/bi00677a022. [DOI] [PubMed] [Google Scholar]
- Swaminathan V., Sundaralingam M. The crystal structures of metal complexes of nucleic acids and their constituents. CRC Crit Rev Biochem. 1979;6(3):245–336. doi: 10.3109/10409237909102565. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., McGhee J. D. DNA-protein interactions. Annu Rev Biochem. 1972;41(10):231–300. doi: 10.1146/annurev.bi.41.070172.001311. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Yamada A., Akasaka K., Hatano H. Proton and phosphorus-31 magnetic relaxation studies on the interaction of polyriboadenylic acid with Mn2+. Biopolymers. 1976 Jul;15(7):1315–1331. doi: 10.1002/bip.1976.360150708. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zimmer C., Luck G., Triebel H. Conformation and reactivity of DNA. IV. Base binding ability of transition metal ions to native DNA and effect on helix conformation with special reference to DNA-Zn(II) complex. Biopolymers. 1974;13(3):425–453. doi: 10.1002/bip.1974.360130302. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


