Abstract
Patients with refractory leukemia or minimal residual disease (MRD) at transplant have increased risk of relapse. Augmentation of irradiation, especially to sites of disease (i.e., bone marrow) is one potential strategy to overcome this risk. We studied the feasibility of radiation dose escalation in high risk patients using total marrow irradiation (TMI) in a phase I dose-escalation trial. Four pediatric and 8 adult patients received conditioning with cyclophosphamide and fludarabine in conjunction with image-guided radiation to the bone marrow at 15 Gy and 18 Gy (in 3 Gy/fractions), while maintaining the total body irradiation (TBI) dose to the vital organs (lungs, hearts, eyes, liver, kidneys) at <13.2 Gy. The biologically effective dose (BED) of TMI delivered to the bone marrow was increased by 62% and 96% at 15 and 18 Gy compared to standard TBI. While excessive dose-limiting toxicity defined by graft failure or excess specific organ toxicity was not encountered, three of six patients experienced treatment-related mortality (TRM) at 18Gy. Thus, we halted enrollment at this dose level and treated an additional 4 patients at 15 Gy. The 1 year OS was 42% (CI 95%, 15–67%) and DFS was 22% (CI 95%, 4–49%). The rate of relapse was 36% (CI 95%, 10–62%) and the non-relapse mortality was 42% (CI 95%, 14–70%). This study shows that dose escalation of TMI to 15 Gy is feasible with acceptable toxicity in pediatric and adult high risk leukemia patients undergoing umbilical cord blood (UCB) and sibling donor transplantation.
Keywords: BMT, Total Marrow Irradiation, cord blood
Introduction
Allogeneic hematopoietic cell transplantation (Allo-HCT) is potentially curative for a variety of malignant disorders. Allo-HCT is typically performed when patients are in remission, and most studies support this practice as outcomes are poor when transplant is performed in relapse. Using CIBMTR registry data, Duval and colleagues showed that patients transplanted in relapse had a three-year event free survival of 16% for acute lymphoblastic leukemia (ALL) or 19% in acute myeloid leukemia (AML)1. Even when allo-HCT is performed in remission, relapse rates vary widely (ranging from 25–40%), depending on factors such as the primary disease, the number of prior remissions, detectable minimal residual disease (MRD) and the intensity of the conditioning regimen.
Over the last 5–10 years, advances in quantitative PCR or multiparameter flow cytometry now allow for the detection of small quantities of MRD in patients who are in morphological remission. This technology has led to a growing appreciation of the variation in leukemic burden prior to allo-HCT in patients who are in remission. Some studies show a strong relationship between pre-transplant MRD and relapse2,3. At present, it is unclear how to reduce relapse risks in patients with active leukemia or detectable pre-transplant MRD4. Using additional chemotherapy prior to transplant to reduce MRD might also be possible and additional pre-transplant chemotherapy risks leukemic progression and/or end organ toxicity which may preclude transplantation or increase treatment related mortality (TRM).
Radiation is an effective component of the transplantation preparatory regimen, both for its immune suppressive properties, as well as for the direct anti-leukemia activity. While leukemia cells from heavily pretreated patients have likely developed chemo-resistance, it is less clear whether this correlates with radiation resistance. Considering that most leukemia patients are radiation naïve, the use of radiation is logical and has been widely used in pre-transplant preparative regimens, especially for patients with lymphoid diseases. One potential method to increase leukemia cell kill might be to augment the dose of irradiation in the preparatory regimen and this, in turn, would be expected to enhance leukemia control5. The higher biological effective dose (BED) associated with total body irradiation (TBI) doses of >13 Gy was significantly correlated with reduced leukemia relapse and/or better disease free survival (DFS)6,7. However, due to the inherent lack of precision of TBI and the sensitivity of vital organs, higher irradiation doses also risk injury to healthy tissues that are not commonly thought to be the main sites of leukemic involvement. Proof of both the benefit and toxicity of higher dose TBI was demonstrated by Clift et al. who randomized patients to receive either standard (12 Gy) or increased dose TBI (15.75 Gy). While the higher radiation dose resulted in reduced relapse, it also increased TRM, resulting in equivalent survival8–10.
This suggests that TBI is limited by the toxicity to vital organs especially lung, liver, eyes, heart and kidneys11–14. While these organs may be involved in the leukemic process, the bone marrow and lymphoid tissue are believed to be the major sites of residual disease in patients needing allo-HCT. With the introduction of helical tomotherapy, a new potential exists to conform the radiation dose to very specific areas of the body, such as the bone marrow. In a preclinical study, using a non-human phantom, we previously showed that helical tomotherapy is accurate and conformal irradiation can be directed to the bone marrow while minimizing the radiation dose to vital organs. In this pre-clinical study of total marrow irradiation (TMI), the average irradiation doses to lungs, heart, eyes, liver, kidneys were reduced by 40–60% compared to conventional TBI. Thus, we hypothesized that it is possible to use TMI to escalate the irradiation dose to the marrow containing spaces (i.e., sites of disease) while maintaining the radiation to vital organs at an acceptable limit (i.e., <13.2 Gy). We tested this hypothesis in a cohort of high risk leukemia patients undergoing myeloablative allo-HCT who were chemotherapy refractory or had pre-transplant MRD.
Patients and Methods
Patient Eligibility and Donor Selection
Patients were eligible if they had adequate performance status (Karnofsky performance status >80% for patients >16 years or Lansky Play Score >50 for younger patients) and acceptable organ function (glomerular filtration rate > 60ml/min/1.73m2, bilirubin, ALT < 5 × upper limit of normal, DLCOcorr > 50% of normal and a left ventricular ejection fraction ≥ 45% by ECHO or MUGA). Patients were eligible if they did not achieve remission with standard induction and salvage chemotherapy or if they had evidence of pre-transplant MRD by 8-color flow cytometry, FISH or cytogenetics. Patients with evidence of pregnancy, HIV infection, uncontrolled serious infection within the last 3 months were excluded from this trial. Allogeneic donors were closely HLA matched umbilical cord blood (UCB) or related donors. This study was approved by the University of Minnesota IRB and were registered as NCT00686556 on clinicaltrials.gov.
Dose escalation and Treatment
The treatment schema is shown in Figure 1A. All patients received fludarabine (25 mg/m2 × 3 consecutive days) and cyclophosphamide (60mg/kg/day IV × 2 days) followed by dose escalated TMI. Details of the TMI technique have been previously described15–17. Briefly, the patient was immobilized using the Body Pro-Lok™ system (CIVCO, Orange City, IA) to assure a consistent positioning during treatment. For pre-treatment planning, a conventional CT, also known as kilovoltage CT (kVCT) scanner (Brilliance CT Big Bore, Philips Healthcare, Cleveland, OH) was used to acquire images. The bony anatomy was contoured in four regions: (i) bones of the skull, (ii) thoracic bones, (iii) upper extremities, and (iv) pelvis. These were used to calculate the clinical target volume (CTV). To account for day-to-day variability during pre-irradiation patient positioning within the tomotherapy device and positional movement of breathing during irradiation, a planning target volume (PTV) was generated with margins (5 mm to 15 mm) around CTV depending on skeletal site. The margins were set by taking into consideration the anatomical region, variations in the precise localization of individual regions before each TMI treatment delivery, using course mode (lower resolution) of MVCT imaging to scan the whole body. The resulting images and contours were then transferred to the Tomotherapy HiArt Planning Station (Tomotherapy, Inc., Madison, WI). An optimal treatment plan was created to deliver the prescribed dose (300 cGy/fraction in 5 or 6 fractions) to the PTV and the reduced radiation dose to vital organs, as described previously17. The rationale for the selection of 3 Gy/fraction was derived from our previously study using TMI simulation, where we considerd the lungs as single most vital organ for toxicity16. In that study the acheivabale mean lung dose was ~50–55 % of the prescribed bone marrow dose. Thus, to keep mean lung dose ≤1.65 Gy/fraction (equivalent to mean lung dose for conventional TBI), we could deliver 3 Gy/fraction to the bone marrow. In addition, in our dose escalation strategy the total lung dose never exceed standard TBI dose of 13.2 Gy. Dose volume histograms (DVHs) were calculated for the target (bone marrow) and vital organs. To increase safety and precision, the patient was scanned with the onboard whole body (WB) megavoltage CT (MVCT) prior to each treatment for fine adjustments to patient positioning (Tomotherapy, Inc, Madison, WI)18. General anesthesia was used for pediatric patients unable to follow commands due to the risk of large body motion which could result in delivery of irradiation to incorrect anatomical sites. A technique was previously developed to use WB MVCT images to calculate the irradiation dose delivered during TMI, taking into consideration the subtle change in patient positioning and was implemented in this trial19,20. The delivered skeletal and pulmonary doses were verified with the original treatment plan. Lastly, due to the lack of critical organs in the lower extremities, they were treated using intensity modulated radiation therapy by the helical tomotherapy machine or using anterior-posterier opposed fields by a linear accelerator.
Figure 1. Schematic representation dose escalation schema.
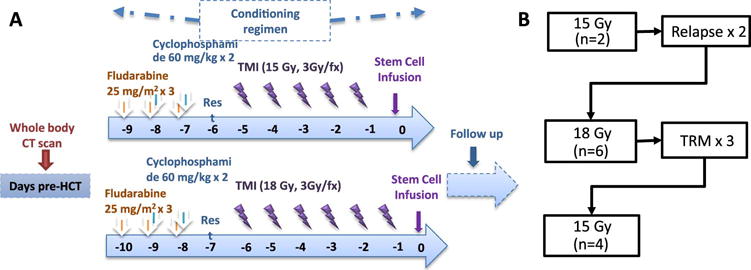
A. All patients received fludarabine and cyclophosphamide and escalating doses of total marrow irradiation. B. Steps of dose escalation. Nine out 12 patients received UCB grafts and 3 others received peripheral lood stem cells.
Calculation of Biological effective dose (BED)
To determine the BED, linear quadratic modeling was used where BED = nd(1+d/(α/β) [as described in21], where d= dose per fraction, n=number of fractions, the “α/β” is termed as “intrinsic radio sensitivity”, higher α/β (~10) indicates rapid proliferation, whereas lower α/β, is appropriate for slowly proliferating tissue. We calculated the BED using α/β value as 1.49 and 3.12, as has been measured in acute myeloid leukemia samples collected from patients at diagnosis22. The effect of dose fractionation on the BED was calculated and the BED ratio (BED-TMI/BED-TBI) was presented to assess the relative increase in BED compared to the conventional TBI.
Study Design and Statistical Analysis
A total of 12 patients were enrolled and treated on this phase I dose-escalation trial. The study was initially designed using a modified continual reassessment method using cohorts of two patients prior to reassessment of assigned radiation dose.23 Dose radiation levels of 12 Gy (dose level -1), 15 Gy (dose level 1), 18 Gy (dose level 2) and 21 (dose level 3) Gy and 24 Gy (dose level 4) were to be tested for a target dose limiting toxicity (DLT) rate of 15%. Dose limiting toxicity was defined as failing to achieve an absolute neutrophil count greater than 500/uL of donor origin by day 42 post-transplant. Overall survival and disease-free survival were estimated using the Kaplan-Meier method24. Relapse, non-relapse mortality, acute and chronic graft vs. host disease (GVHD) and engraftment were estimated using competing risks methods25. For dosimetry comparison by organ to 13.2 Gy, a one-proportion t-test was applied without adjustment for multiple comparisons. SAS 9.3 (SAS Institute, Cary, NC) was used to perform all statistical analyses.
Results
TMI Dose Escalation
Demographic and characteristics of the participants are shown in Table 1. A total of 12 patients were enrolled on this dose escalation study to determine the safety and tolerability of dose escalated, image guided TMI. Of these patients, 4 were <18 yrs of age and 10 had acute lymphoid leukemia. At the time of transplant, 4 patients had active leukemia and 8 had detectable MRD by flow cytometry and/or cytogenetics. Nine patients received UCB, while the remainder were transplanted with filgrastim-mobilized blood stem cells from matched related adult donor sources. Dose escalating TMI was given following myeloablative chemotherapy with fludarabine and cyclophosphamide (Figure 1A). Two patients were treated at dose level one (15 Gy). Both tolerated the preparative regimen without complications and subsequently 6 patients were enrolled in the next dose cohort (18 Gy). Three of six patients had TRM (days 80, 7 and 79 post-HCT) which were not attributed to the conditioning, however, and it was elected to halt enrollment at this dose level. An additional 4 patients were enrolled at 15 Gy (Figure 1B). The timing and nature of the targeted toxicities are shown in table 2. The 1 year OS for the whole cohort (15 and 18 Gy) was 42% (95 %CI, 15–67%) and DFS was 22% (95% CI, 4–49%) (Figure 2A and B). At 1 year, the rate of relapse was 36% (95% CI, 10–62%) and the non-relapse mortality was 42% (95% CI, 14–70%) (Figure 2C and D); 33% relapse and 17% TRM in the 6 receiving 15 Gy. Of note, all cases of relapse occurred in the blood and/or bone marrow and there were not extramedullary relapses observed. Causes of death are listed in table 1 and include graft failure (n=1), fungal infection (n=1), ARDS (n=2), cardiac failure (n=1), and disease recurrence (n=4).
Table 1.
Demographic and clinical characteristics.
| Patient | Age at Transplant | Diagnosis | Disease Status at Transplant | Dose | Stem Cell Source | Survival Status, DOD | Primary COD |
|---|---|---|---|---|---|---|---|
| 1 | 6.9 | ALL | Relapse 1 | 15 Gy | UCB | Dead, d65 | Leukemia relapse |
| 2 | 2.0 | ALL | Relapse 1 | 15 Gy | UCB | Dead, d99 | Leukemia relapse |
| 3 | 31.8 | ALL | Relapse 1 | 18 Gy | UCB | Dead, d58 | Graft Failure |
| 4 | 25.7 | ALL | CR2, MRD+ | 18 Gy | UCB | Dead, d935 | Leukemia relapse |
| 5 | 37.3 | ALL | Relapse 1 | 18 Gy | UCB | Dead, d80 | ARDS |
| 6 | 20.8 | ALL | CR2, MRD+ | 18 Gy | UCB | Dead, d784 | Leukemia relapse |
| 7 | 8.4 | AML | PIF | 18 Gy | BM | Dead, d7 | Rhizopus Infection |
| 8 | 24.1 | ALL | CR1, MRD+ | 18 Gy | UCB | Dead, d79 | ARDS |
| 9 | 8.4 | ALL | CR2, MRD+ | 15 Gy | UCB | Alive, d524+ | . |
| 10 | 54.5 | AML | CR4, MRD+ | 15 Gy | PBSC | Dead, d86 | Cardiac Failure |
| 11 | 26.7 | ALL | CR1, MRD+ | 15 Gy | PBSC | Alive, d414+ | . |
| 12 | 47.0 | PL | CR1, MRD+ | 15 Gy | UCB | Alive, d272+ | . |
ALL – acute lymphoblastic leukemia pre B cell; AML – acute myeloid leukemia; PL - prolymphocytic leukemia; CR – complete remission; PIF – primary induction failure; Gy – gray; UCB – umbilical cordblood; BM – bone marrow; PBSC – peripheral blood stem cell; COD – cause of death; DOD, day of death, ARDS – acute respiratory distress syndrome, d – day post-transplant
Table 2.
Targeted Toxicities, Grade and Days Post-Transplant
| AE | Grade 2 (n) | Day post-transplant (days) | Grade 3 | Day post-transplant (days) |
|---|---|---|---|---|
| Nausea | 3 | 7, 7, 7 | 7 | 7, 7,30, 30, 30, 30, 100 |
| Fatigue | 4 | 7, 7, 7, 7 | ||
| Muscle weakness | 3 | 7, 7, 7 | 1 | 100 |
| Musculoskeletal pain | 2 | 7, 100 | ||
| Diarrhea | 2 | 7, 180 | ||
| Osteonecrosis | 1 | 30 | ||
| Pain (any site) | 6 | 7, 7, 7, 7, 30 100 | 2 | 7, 30 |
| Rash | 2 | 7, 30 | ||
| Vomiting | 7 |
Figure 2. Clinical outcomes.
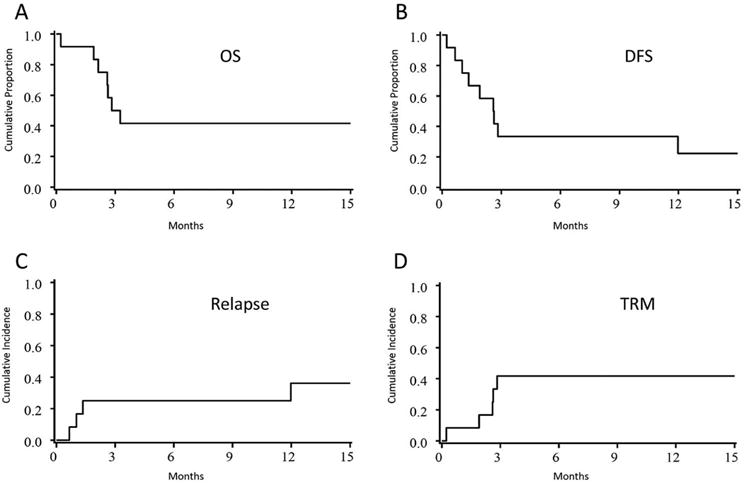
A) overall survival (OS), B) disease free survival (DFS), C) relapse and D) treatment related mortality (TRM).
Considering that increased irradiation dose to the BM could impact hematopoietic cell engraftment and that patients were treated mainly with umbilical cord blood (n=9), we examined the probability and kinetics of neutrophil engraftment. There was one non-engraftment event. The cumulative incidence of engraftment by day 42 was 89% (95% CI, 66–99%) and the median time to neutrophil recovery was 26 days. The day 100 grade II–IV acute graft-versus-host disease (GVHD) incidence was 33% (95% CI, 7–59%); grade III–IV 25% (95% CI, 1–49%). The incidence of neutrophil engraftment and aGVHD were similar to contemporaneous and historical controls26–28.
Dosimetry to Bone Marrow and Organs
The goal of the study was to determine whether it was possible to safely augment the TMI radiation dose to the bones (and associated bone marrow), while delivering a TBI-equivalent dose (of 13.2 Gy) or less to the rest of the body. Figure 3A shows a representative irradiation dose distribution map using dose painting in a patient who received 15 Gy TMI, showing the targeting of the marrow spaces and the sparing of the non-hematopoietic tissues. In Figure 3B the mean organ irradiation dose is shown at 15 Gy and 18 Gy dose levels and is compared to conventional TBI of 13.2 Gy. For all patients, the total dose to lung was <10 Gy and lung dose/fraction was also kept ≤1.65 Gy. For both 15 Gy and 18 Gy cohorts, the dose to the vital organs were significantly reduced to 10% to 80% of the marrow dose. A statistically significant reduction from 13.2 Gy was seen in the eyes, lens, oral cavity, parotids, heart, lungs, liver, stomach, peritoneum, kidneys and bladder at 15 Gy and in the eyes, lens, parotids, heart, lungs and kidney at 18 Gy (all p<.05 compared to TBI). Both 15 and 18 Gy doses statistically significantly increased in the bone marrow. Figure 3C depicts the planned and delivered irradiation doses to the bone marrow and lungs for patients in the 15 Gy group, showing that both anatomic regions were within 2–3% of prescribed dose.
Figure 3. Radiation dosimetric information.
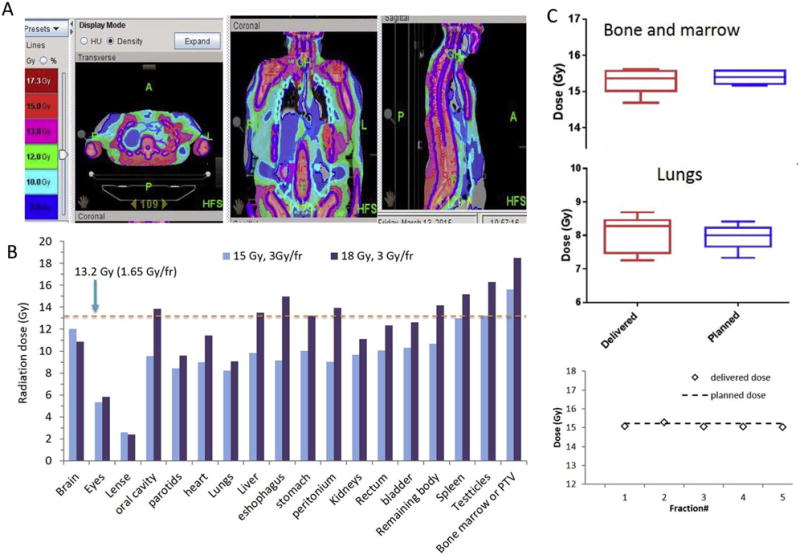
A. dose distribution (representative) for a patient receiving TMI. B. Organ dose comparison between 15 (blue) or 18 (purple) Gy TMI vs conventional 13.2Gy TBI. C. Verification of planned and delivered total radiation dose in bone marrow and lungs (top) and daily TMI delivered dose verification in one representative subject (bottom).
While the total irradiation dose delivered to the patient is an important variable, this does not fully describe the effect of irradiation on both the malignant and healthy tissues21,22. Therefore, other measures have been used to incorporate the total dose of irradiation and the potential to induce cell death, such as the biological effective dose (BED). The main parameters affecting BED are the irradiation dose in each fraction, the number of fractions delivered and the intrinsic radiosensitivity of the tissue. Therefore, following dose escalated irradiation, BED changes differently than does the prescribed (physical) dose. The effect of intrinsic radiosensitivity on BED is small (Figure 4A), however the effect of dose fractionation on BED is large (Figure 4B). When increasing the TMI irradiation dose from 13.2 Gy to 15 Gy, there is a 14% increase, however as shown in Figure 4B, the percent change in BED is 62% (BED ratio 1.6). Likewise, the percent increase in the total dose irradiation from 13.2 Gy to 18 Gy is a 36% increase, while the percent change in BED is 96% (BED ratio 1.9). Thus, while the increase in physical dose delivered was relatively small, the BED was considerably higher due to the relatively large irradiation dose delivered in each fraction.
Figure 4. The biological effective dose (BED) of TMI dose esclation.
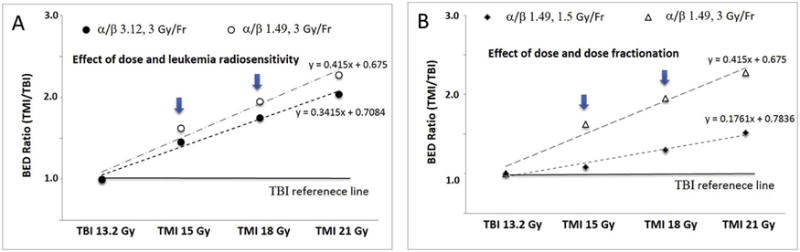
A. Effect of radiosensitivity on BED while escalating radiation dose. B. Effect of dose fractionation on BED while escalating radiation dose.
Discussion
We report the outcomes of a novel approach to increase the irradiation component of a myeloablative conditioning regimen. We found that dose escalation was feasible with highly focused radiation targeted to the bone marrow. The goal was to escalate the irradiation delivered to the marrow containing spaces, while maintaining the dose of irradiation to <13.2 Gy for other organs. We used image guided tomotherapy to identify and irradiate the marrow spaces. Based on the study design of delivering 3 Gy fractions, we show that while the augmentation in the radiation dose was relatively minimal (13.2 to 15 Gy), the BED delivered to the tissues and residual leukemia was substantial. The DFS and relapse rates were similar to historical controls in high risk or relapsed leukemia patients1.
Recently, other investigators have also explored the use of TMI in preparative regimens29–31. The timing of dose-escalated irradiation relative to chemotherapy seems to be an important consideration as in some studies, dose escalation was feasible and well tolerated, while in others dose-limiting toxicities were striking. For example, Stein and coworkers summarized the outcomes of two different conditioning regimens using dose-escalated TMI. Interestingly, the sequence of irradiation and chemotherapy differed. The first regimen used TMI followed by chemotherapy (etoposide and cyclophosphamide), while the second gave chemotherapy (busulfan) before TMI, followed by a single dose of etoposide. While the chemotherapeutic agents were different, the first trial was well tolerated, with dose escalation up to 15Gy, while the second resulted in significant, grade 4 dose limiting toxicities. Similarly, Patel et al used a linac-based TMI method where irradiation and fludarabine were given simultaneously, followed by Busulfan31. These investigators found dose limiting toxicities at 12 Gy and concluded that 9 Gy was the MTD. Similar to these studies, we gave chemotherapy prior to TMI and found that the 18 Gy dose was toxic, but not strictly dose-limiting. We interpret these varying results to suggest that delivering irradiation prior to chemotherapy might allow for safer irradiation escalation. In support of this, Stein and coworkers have recently reported in abstract form, the escalation of TMI to 20 Gy followed by cyclophosphamide and etoposide30. It is also worth noting that their dose escalation strategy (to 20 Gy) used 2 Gy/fraction and thus an increase in BED of 68% relative to conventional TBI (BED ratio 1.7). We increased the BED to a similar amount, by giving a overall lower total dose, but more radiation in each fraction. Therefore, our method could be considered as an alternative strategy to enhance biological effecetive dose.
This report highlights two unique aspects of radiation escalation strategies: i) improving precision and accuracy of radiation delivery and ii) delivery of higher irradiation dose per fraction to enhance the BED. We used TMI to deliver highly focused radiation to the skeleton, which is a large and complex anatomical structure. Given this, it was essential to assure that patient positioning within the irradiation unit was accurate over the course of multiple treatments lasting for either 5 or 6 days in both small children and large adults which requires careful attention to patient positioning with each treatment19. Secondly, the BED provides a biological equivalent reference to compare the various strategies of TMI dose escalation with standard TBI. In our view this is essential as different clinical studies prescribe differing total (physical) doses that vary in the dose per fraction and these variations have a large effect on BED. The increasing dose per fraction (also called “hypofraction”) may offer a clinical benefit for TMI. These concepts and clinical benefit was already reported in prostate cancer32. This characteristic would imply a unique opportunity to improve the therapeutic ratio by using TMI to treat the patient with fewer, but larger fractions of radiation. However, a future comparative clinical trial would be needed to define the efficacy of the two approaches.
There are several technological limitations of this study and potential confounding by the patients selected for study. While we found TMI to be precise, the time required to plan the treatment and verifications for each patient was considerable (~40–50 hours) when we started the program. A fast treatment delivery verification process significantly reduced the dosimetric verification time33 As well, the time for each delivery session took ~45–60 minutes per fraction. To overcome the prolonged delivery time, two rapid imaging methods are being developed by our group and will be incorporated in future trials34,35. Another important technological limitation is the ability to verify the dose delivered to the patient, an essential component for precise radiation medicine. We utilized the onboard MVCT to measure the delivered irradiation dose. Further improvements to incorporate dosimetric accuracy are ongoing within a multi-center study and these include characterization of 3D anatomical changes during therapy and how this impacts treatment delivery. Additional radiobiologic considerations used the α/β value derived from AML patients which, may not represent lymphoid leukemia patients, who were the majority of subjects on this study. We plan to measure α/β value of leukemia from bone marrow samples at the time of diagnosis to better understand the sensitivity of specific leukemias. Lastly, our patient population was heterogeneous, with most being heavily pre-treated thus suggesting caution in generalization about the tolerability of this augmented conditioning regimen. Moreover, at 18 Gy, we observed 2 episodes of ARDS, however the dose of irradiation to the lungs was kept belwo 10 Gy, hence we attribute this to a heavily pre-treated, older population that is prone to high TRM. We also report the first experience with TMI in pediatric patients. Given the inability of young children to remain inactive for the entire treatment, anesthesia support was required. While this clearly increases some complexity and risks, future studies need to address the benefits of this approach as vital organs sparing techniques may have particularly important long term benefits for pediatric patients.
It is unknown whether increased radiation could damage the bone marrow microenvironment and compromise hematopoietic cell engraftment. Our prior study indicates that higher doses of irradiation could lead to residual damage to the bone marrow following myeloablative (vs. reduced intensity) conditioning36. By extrapolation, dose escalated TMI might damage marrow stroma even further. During our initial study development, there was concern about the high dose rate (400–800 cGy/min) of radiation delivery used during TMI. In a preclinical zebrafish allo-transplant model, recipients conditioned with the higher dose rate associated with TMI showed significantly improved donor-derived engraftment (p≤0.0001)37 supporting the preclinical rationale for our study. All but one evaluable patient had neutrophil recovery in an acceptable time frame. Moreover, we have not observed late effects and longer follow-up is certainly needed. Future modifications will include murine TMI models to interrogate the kinetics of engraftment, efficacy of antileukemia activity, the effect of dose fractionation, and chemo/XRT ordering on transplant outcomes.
Key points.
It was feasible to escalate irradiation to 15 Gy using TMI. Further escalation to 18 Gy was not possible, due to combined toxicities of irradiation and chemotherapy.
The biological effective dose (BED) delivered to the bone marrow were substantially increased in TMI while the actual irradiation dose was similar to TBI.
Acknowledgments
This work was supported by the National Institute of Health grants (1R01CA154491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
Author Contribution:
SH: conceived of irradiation technique, wrote protocol, delivered irradiation, collected and analyzed data and wrote manscript
CB, SGH, VB, CU and DW: assisted in writing the clinical protocol, enrolled patients, assisted in writing manuscript
TD: Oversaw all aspects of statistical analysis and dose escalation and assisted in writing manuscript
YT, CW, DZ: assisted with irradiation conceptual design, deliver and interpretation and assisted in writing manuscript
KD: oversaw all aspects of irradiation delivery and assisted in writing manuscript
MRV: wrote protocol, collected and analyzed data, wrote protocol and manuscript and enrolled patients.
References
- 1.Duval M, Klein J, He W, et al. Hematopoietic Stem-Cell Transplantation for Acute Leukemia in Relapse or Primary Induction Failure. Journal of clinical oncology. 2010;28(23):3730. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulsipher MA, Carlson C, Langholz B, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501–3508. doi: 10.1182/blood-2014-12-615757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisdorf DJ, Millard HR, Horowitz MM, et al. Allogeneic Transplantation for Advanced AML: The Value of Complete Remission. Cancer. 2016 doi: 10.1002/cncr.30536. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheldon TE. The radiobiological basis of total body irradiation. Br J Radiol. 1997;70(840):1204–1207. doi: 10.1259/bjr.70.840.9505837. [DOI] [PubMed] [Google Scholar]
- 6.Kal HB, Loes van Kempen-Harteveld M, Heijenbrok-Kal MH, Struikmans H. Biologically effective dose in total-body irradiation and hematopoietic stem cell transplantation. Strahlenther Onkol. 2006;182(11):672–679. doi: 10.1007/s00066-006-1528-6. [DOI] [PubMed] [Google Scholar]
- 7.Marks D, Forman S, Blume K, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biology of Blood and Marrow Transplantation. 2006;12(4):438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871. [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660–1665. [PubMed] [Google Scholar]
- 10.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92(4):1455–1456. [PubMed] [Google Scholar]
- 11.Belkacemi Y, Ozsahin M, Pene F, et al. Cataractogenesis after total body irradiation. Int J Radiat Oncol Biol Phys. 1996;35(1):53–60. doi: 10.1016/s0360-3016(96)85011-5. [DOI] [PubMed] [Google Scholar]
- 12.Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys. 1995;31(5):1249–1256. doi: 10.1016/0360-3016(94)00428-N. [DOI] [PubMed] [Google Scholar]
- 13.Keane TJ, Van Dyk J, Rider WD. Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int J Radiat Oncol Biol Phys. 1981;7(10):1365–1370. doi: 10.1016/0360-3016(81)90032-8. [DOI] [PubMed] [Google Scholar]
- 14.Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28(3):283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 15.Hui S, Verneris M, Higgins P, et al. Helical tomotherapy targeting total bone marrow-First clinical experience at the University of Minnesota. Acta Oncologica. 2007;46(2):250–255. doi: 10.1080/02841860601042449. [DOI] [PubMed] [Google Scholar]
- 16.Hui SK, Kapatoes J, Fowler J, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(10):3214–3224. doi: 10.1118/1.2044428. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Verneris MR, Dusenbery KE, et al. Peripheral dose heterogeneity due to the thread effect in total marrow irradiation with helical tomotherapy. Int J Radiat Oncol Biol Phys. 2013;87(4):832–839. doi: 10.1016/j.ijrobp.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruchala KJ, Olivera GH, Kapatoes JM, Schloesser EA, Reckwerdt PJ, Mackie TR. Megavoltage CT image reconstruction during tomotherapy treatments. Phys Med Biol. 2000;45(12):3545–3562. doi: 10.1088/0031-9155/45/12/303. [DOI] [PubMed] [Google Scholar]
- 19.Hui S, Verneris M, Froelich J, Dusenbery K, Welsh J. Multimodality image guided total marrow irradiation and verification of the dose delivered to the lung, PTV, and thoracic bone in a patient: a case study. Technology in cancer research & treatment. 2009;8(1):23. doi: 10.1177/153303460900800104. [DOI] [PubMed] [Google Scholar]
- 20.Hui SK, Lusczek E, DeFor T, Dusenbery K, Levitt S. Three-dimensional patient setup errors at different treatment sites measured by the Tomotherapy megavoltage CT. Strahlenther Onkol. 2012;188(4):346–352. doi: 10.1007/s00066-011-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 22.Cowen D, Richaud P, Landriau S, et al. Radiobiological features of acute myeloblastic leukemia: comparison of self-renewal versus terminally differentiated populations. Int J Radiat Oncol Biol Phys. 1994;30(5):1133–1140. doi: 10.1016/0360-3016(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 23.Goodman SN, Zahurak ML, Piantodosi S. Some practical improvements in the continual reassessment method for phase I studies. Statistics in Medicine. 1995;14(11):1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53(282):457–481. [Google Scholar]
- 25.Lin D. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in medicine. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner JE, Jr, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685–1694. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corvo R, Zeverino M, Vagge S, et al. Helical tomotherapy targeting total bone marrow after total body irradiation for patients with relapsed acute leukemia undergoing an allogeneic stem cell transplant. Radiother Oncol. 2011;98(3):382–386. doi: 10.1016/j.radonc.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Stein A, Palmer J, Tsai NC, et al. Phase I Trial of Total Marrow and Lymphoid Irradiation Transplant Conditioning in Patients with Relapsed/Refractory Acute Leukemia. Biol Blood Marrow Transplant. 2017;23(4):618–624. doi: 10.1016/j.bbmt.2017.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P, Aydogan B, Koshy M, et al. Combination of linear accelerator-based intensity-modulated total marrow irradiation and myeloablative fludarabine/busulfan: a phase I study. Biol Blood Marrow Transplant. 2014;20(12):2034–2041. doi: 10.1016/j.bbmt.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late-responding normal tissue. International Journal of Radiation Oncology* Biology* Physics. 2002;52(1):6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Hui SK. Fast, simple, and informative patient-specific dose verification method for intensity modulated total marrow irradiation with helical tomotherapy. Radiat Oncol. 2014;9:34. doi: 10.1186/1748-717X-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magome T, Haga A, Takahashi Y, Nakagawa K, Dusenbery KE, Hui SK. Fast Megavoltage Computed Tomography: A Rapid Imaging Method for Total Body or Marrow Irradiation in Helical Tomotherapy. Int J Radiat Oncol Biol Phys. 2016;96(3):688–695. doi: 10.1016/j.ijrobp.2016.06.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi Y, Vagge S, Agostinelli S, et al. Multi-institutional feasibility study of a fast patient localization method in total marrow irradiation with helical tomotherapy: a global health initiative by the international consortium of total marrow irradiation. International Journal of Radiation Oncology* Biology* Physics. 2015;91(1):30–38. doi: 10.1016/j.ijrobp.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilke C, Holtan SG, Sharkey L, et al. Marrow damage and hematopoietic recovery following allogeneic bone marrow transplantation for acute leukemias: Effect of radiation dose and conditioning regimen. Radiotherapy and Oncology. 2016;118(1):65–71. doi: 10.1016/j.radonc.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass TJ, Hui SK, Blazar BR, Lund TC. Effect of radiation dose-rate on hematopoietic cell engraftment in adult zebrafish. PloS one. 2013;8(9):e73745. doi: 10.1371/journal.pone.0073745. [DOI] [PMC free article] [PubMed] [Google Scholar]


