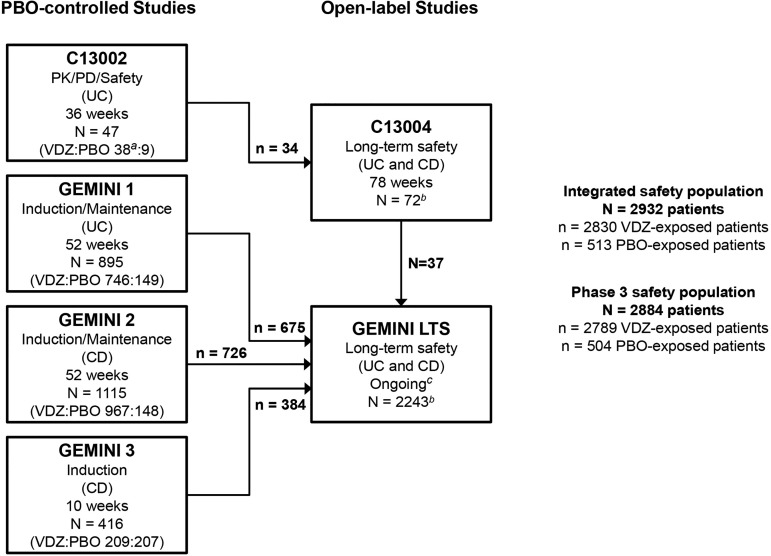
Figure 1.
Patient distribution within the overall safety population. The overall safety population includes all patients who received ≥1 dose of study drug in the six studies. The phase 3 safety population includes patients from the phase 3 GEMINI studies only. Patients randomised to PBO in GEMINI 1, GEMINI 2 or GEMINI 3 and who enrolled into GEMINI LTS received open-label VDZ in that study. Thus, VDZ-exposed patients may also have been exposed to PBO. CD, Crohn's disease; LTS, long-term safety; PBO, placebo; PD, pharmacodynamics; PK, pharmacokinetics; UC, ulcerative colitis; VDZ, vedolizumab. aOne enrolled patient randomised to VDZ was not dosed. bIncludes 38 and 421 VDZ-naïve patients who enrolled directly into C13004 or GEMINI LTS, respectively. cIncludes data collected from 22 May 2009 to 27 June 2013.