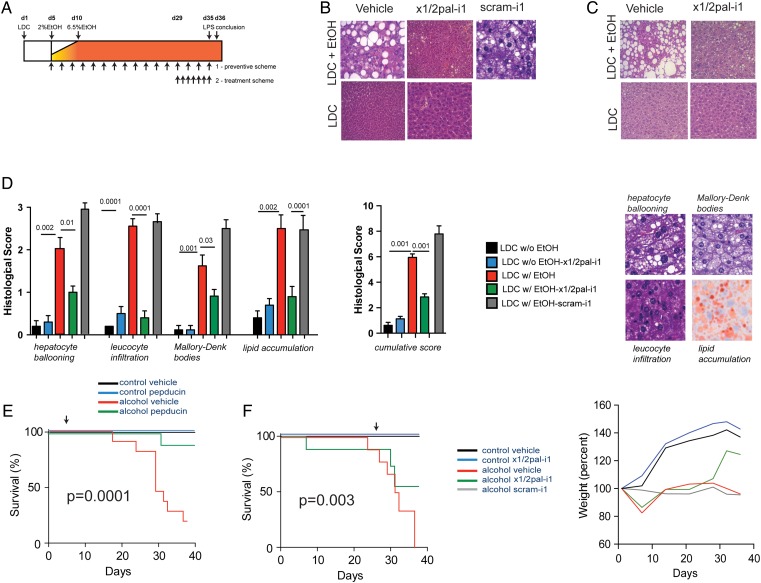
Figure 1.
x1/2pal-i1 treatment inhibited the development and progression of alcoholic liver disease. (A) Experimental approach. Mice received the Lieber–DeCarli (LDC) diet. Pepducin therapy was commenced either with the introduction of ethanol (day 5; ‘preventative setting’; 2.5 mg/kg x1/2pal-i1 subcutaneous every other day) or after mice had established disease (day 29, ‘therapeutic setting’; 5.0 mg/kg x1/2pal-i1 subcutaneous once a day). On day 35, mice received LPS (2.5 mg/kg intraperitoneal) and were assessed 24 h later. (B) x1/2pal-i1 prevents development of liver steatosis. Representative liver sections stained with H&E (n=15). (C) Therapeutic x1/2pal-i1 reverts liver steatosis. Representative liver sections stained with H&E (n=15). (D) Histological disease activity. H&E-stained sections for hepatocyte ballooning, leucocyte infiltration and Mallory–Denk bodies, Oil-red-O for steatosis. Statistical analysis: Mann–Whitney U after Kruskal–Wallis; n=15 per group; *p<0.05 (LDC vs LDC-EtOH); †p<0.05 (LDC-EtOH vs LDC-EtOH-x1/2pal-i1). (E) x1/2pal-i1 treatment prevents from alcohol-induced mortality. Mice received 2.5 mg/kg of x1/2pal-i1, scram-i1 (2.5 mg/kg) or vehicle control every other day. Statistical analysis: Mantel–Cox test p<0.0001. n=15. (F) x1/2pal-i1 therapy reduces alcohol-induced mortality and prevents from weight loss. Mice received 5 mg/kg of x1/2pal-i1, scram-i1 or vehicle control every day from day 29 until the conclusion of the experiment. The animals’ weight was taken every other day and weight curves were compared. Statistical analysis: Mantel–Cox test p<0.0001. n=15 (n=7 for scram-i1).