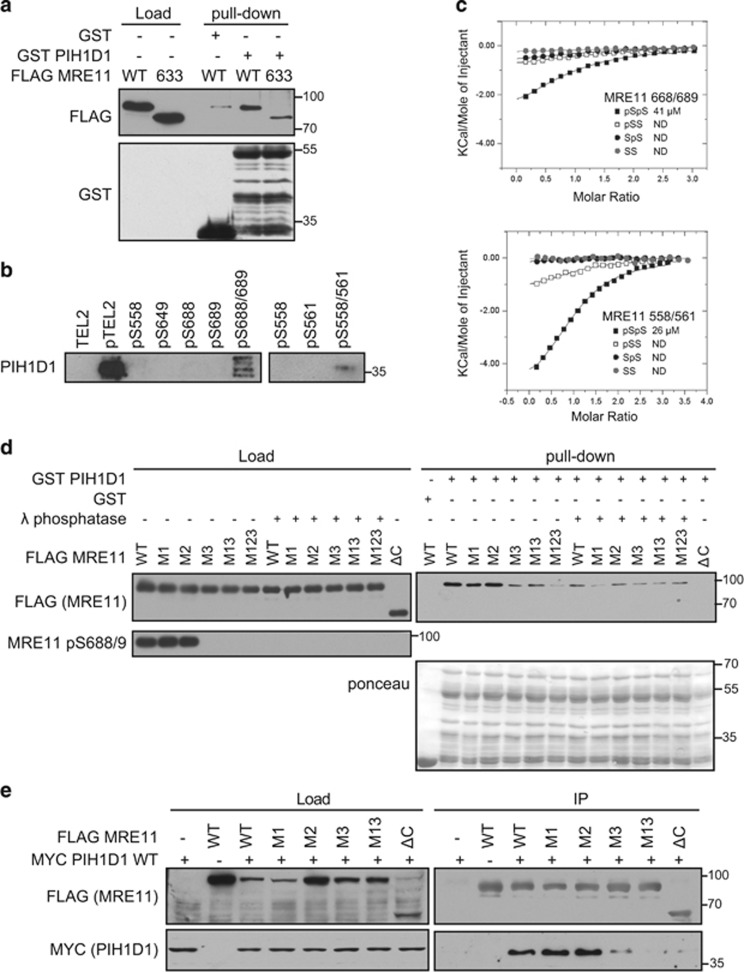
Figure 2.
Phosphorylated serine 558/561 and 688/689 of MRE11 interact directly with PIH1D1. (a) MRE11 ATLD1 truncation reduces binding of PIH1D1. GST or GST PIH1D1 WT were bound to glutathione sepharose beads (GenScript, Piscataway, NJ, USA) for 2 h at 4 °C, washed with TBS-Tween (150 mM NaCl, 3 mM KCl, 25 mM Tris-HCl (pH 7.2), 10% glycerol) and incubated with 90μl of eluted FLAG MRE11 WT or FLAG MRE11 truncated at residue 633 for 1h at 4 °C. The beads were washed 4x with TBS-Tween and boiled in 2xLSB buffer (100 mm Tris (pH 6.8), 200 mm dithiothreitol (DTT), 4% sodium dodecyl sulfate (SDS), 0,2% bromophenol blue, 20% (v/v) glycerol). Bound proteins were analyzed by immunoblotting. Recombinant full-length GST PIH1D1 is at mark 55 kDa, and other bands are degradation products. (b) Biotinylated peptides containing a double phosphorylation of serine 558/561 or 688/681 pull-down PIH1D1. Biotinylated peptides were synthesized by the Francis Crick Institute Peptide Chemistry facility and peptide pull-down from HeLa nuclear extract (4 mg/ml; Ipratech, Mons, Belgium) was performed as described previously.20 Bound PIH1D1 was analyzed by immunoblotting. Phosphorylated and non-phosphorylated TEL2 peptides were used as positive and negative controls, respectively. (c) Isothermal titration calorimetric (ITC) analysis of MRE11 binding (see Supplementary Tables S2a and S2b for sequences) to PIH1D1 1–180. Purification of PIH1D1 1–180 PIH-N domain fragment and ITC was performed as described previously. (d) Phosphorylation of serine 688/689 is required for interaction between MRE11 and PIH1D1. GST or GST PIH1D1 WT were incubated with purified FLAG MRE11 WT or mutants M1, M2, M3, M13, M123 and interaction was assessed by immunoblotting against FLAG. Where indicated, purified MRE11 was treated with λ phosphatase prior the pull-down assay. Staining with MRE11 pS688/9 antibody was used as control of efficient dephosphorylation. Recombinant full-length GST PIH1D1 is at mark 55 kDa, and other bands are degradation products. (e) Interaction between MRE11 and PIH1D1 in cells depends on serine 688/689. HCT116 cells were co-transfected with plasmids coding MYC PIH1D1 and FLAG MRE11 WT, M1, M2, M3, M13 or MRE11 ΔC mutants. Cell extracts were incubated with anti-FLAG M2 affinity gel and immunoprecipitated proteins were analyzed with MYC (GeneTex, Zeeland, MI, USA; GTX115046) and FLAG antibodies.