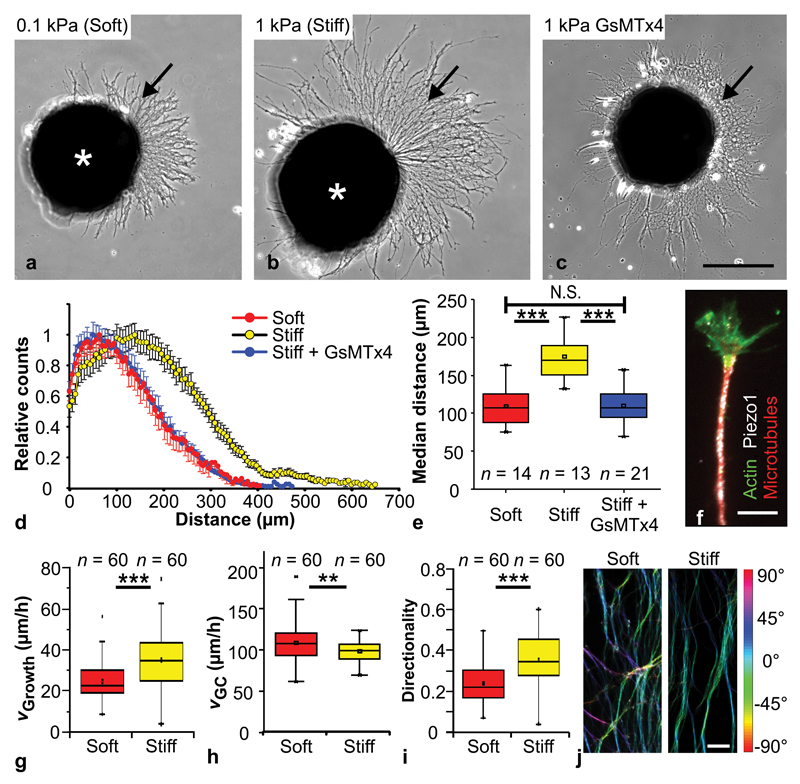
Figure 1. Mechanosensitivity of RGC axons in vitro.
(a, b) Cultures of Xenopus eye primordia (asterisks) on (a) ‘soft’ (0.1 kPa) and (b) ‘stiff’ (1 kPa) substrates. Arrows indicate axons. (c) Eye primordium grown on a stiff substrate and treated with GsMTx4, a blocker of mechanosensitive ion channels. Scale bar: 200 µm. (d) Sholl analysis of axon lengths after 24 hours (normalized counts as mean ± S.E.M.). (e) Median distances shown in (d). Axons were significantly longer on stiffer substrates than either on soft ones (One-Way-ANOVA followed by Bonferroni post-hoc test; P = 2.79 × 10-7, t = 6.354,) or after GsMTx4 treatment (P = 5.01 × 10-8, t = -6.855). Neurons grown on stiff substrates and treated with GsMTx4 resembled neurons grown on soft substrates (P = 1.00, t = 0.082). n = number of eye primordia from three biological replicates. (f) Immunocytochemistry showing f-actin (green), beta-tubulin (red) and the mechanosensitive ion channel Piezo1 (white). Scale bar: 10 µm. (g) The extension velocity of axons was higher on stiff substrates (Mann-Whitney-Test; P = 9.32 × 10-6, Z = 4.432). (h) On soft substrates, growth cones explored their environment more and migrated significantly faster than on stiff ones (two-tailed t-test; P = 0.00867, t = 2.669). (i) On stiff substrates, axon growth was more directed (i.e., straight) than on soft substrates (Mann-Whitney-Test; P = 1.10 × 10-6, Z = 4.873). n = number of axons from three biological replicates. (j) Processed fluorescence images of beta-tubulin-labelled RGC axons; colour represents local angular orientation of axonal segments. On soft substrates, axons grew less directionally persistent (from bottom to top; cf. Supplementary Fig. 2e-g). Scale bar: 15 µm. All experiments were repeated three times, and representative images are shown. Boxes show the 25th, 50th (the median), and 75th percentiles, whiskers the spread of the data.