Figure 1.
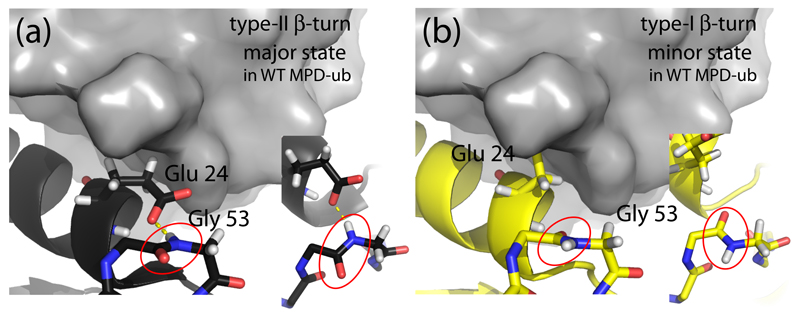
Conformation of ubiquitin in the βI and βII states. (a) The MPD-ub crystal structure, focusing on the β-turn region and the adjacent α-helix. A neighboring molecule in the crystal is shown in surface representation in grey. The zoom (right insert in (a)) shows a top view of the peptide plane Asp 52/Gly 53 (red ellipse). Note the hydrogen bond to the side chain of Glu 24. This β-turn conformation is called type-II β-turn. (b) Structure of a ubiquitin chain in type-I β-turn conformation (PDB entry 1UBI, yellow) placed inside the crystal arrangement of MPD-ub, by structural alignment. The most striking difference between type-II (a) and type-I (b) structures are the orientation of the peptide plane Asp 52/Gly 53, and the orientation of Glu 24’s side chain. In βI conformation (b) the side chain is rotated outward while it points to Gly 53 in βII. In the context of the crystal packing of MPD-ub, rotating Glu 24 outward would result in a steric clash. This explains why the βI conformation is energetically unfavorable in MPD-ub, whereas in solution and most other crystals βI is favored.