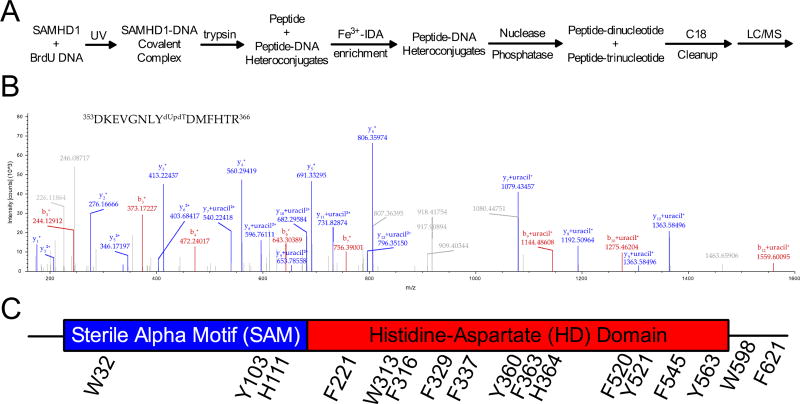
Figure 2. Mass spectrometry for the identification of BrdU crosslinks to SAMHD1.
(A) A schematic for mass spectrometry sample preparation. (B) A representative MS2 spectrum from a cross-linked peptide. The parent ion m/z corresponded to the shown peptide with an attached dUpdT dinucleotide and the shown fragment ions unambiguously identify Y360 as the crosslinked residue. Upon fragmentation, b and y ions of the peptide were observed, and the di- or trinucleotide modification fragmented to uracil. (C) All of the cross-linked amino acids observed in the mass spectrometry studies are shown on the linear domain architecture of SAMHD1.