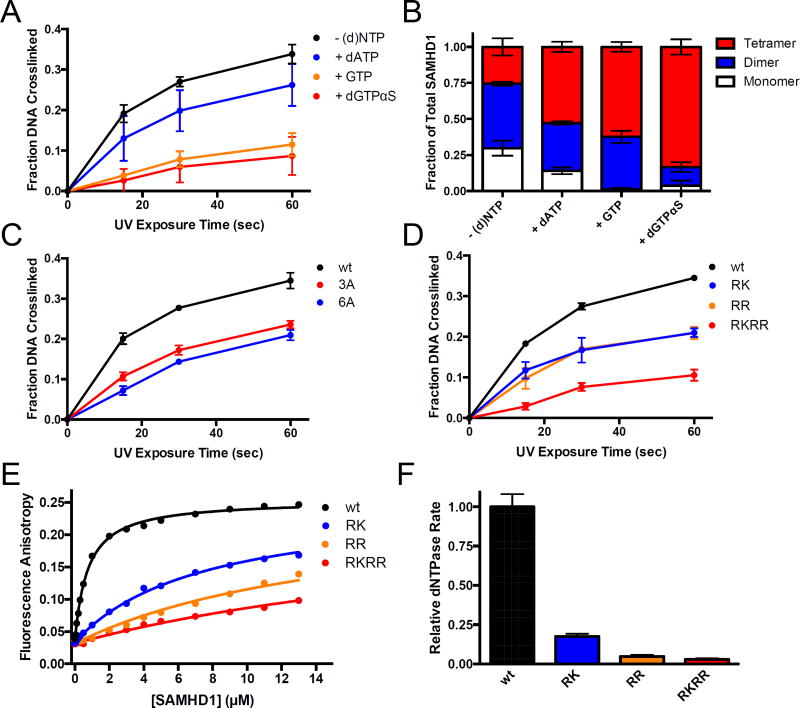
Figure 4. Biochemical validation of the mass spectrometry crosslinking results.
(A) BrdU crosslinking reactions were performed with SAMHD1 in the presence of dATP, GTP, or dGTPαS to induce oligomerization (1 mM each) and the fraction of total DNA crosslinked to SAMHD1 was quantified. (B) The oligomeric state of SAMHD1 under the conditions in (A) was assessed by glutaraldehyde crosslinking and quantified by densitometry. (C) BrdU crosslinking reactions were performed with wild-type SAMHD1, or the 3A and 6A alanine mutants (see text) and the fraction of the total DNA cross-linked to the protein was quantified. (D) BrdU crosslinking reactions with wild-type SAMHD1 or the indicated glutamate charge-reversal mutants RK, RR, and RKRR (see text). The fraction of the total DNA cross-linked to the protein at each time point was quantified by densitometry. (E) Increasing anisotropy of a fluorescein-labeled dT60 DNA oligonucleotide as a function of added wt or mutant SAMHD1. (F) The normalized dNTP hydrolase activity of wt SAMHD1 and the indicated mutants was determined using 1 mM dGTP as the substrate.