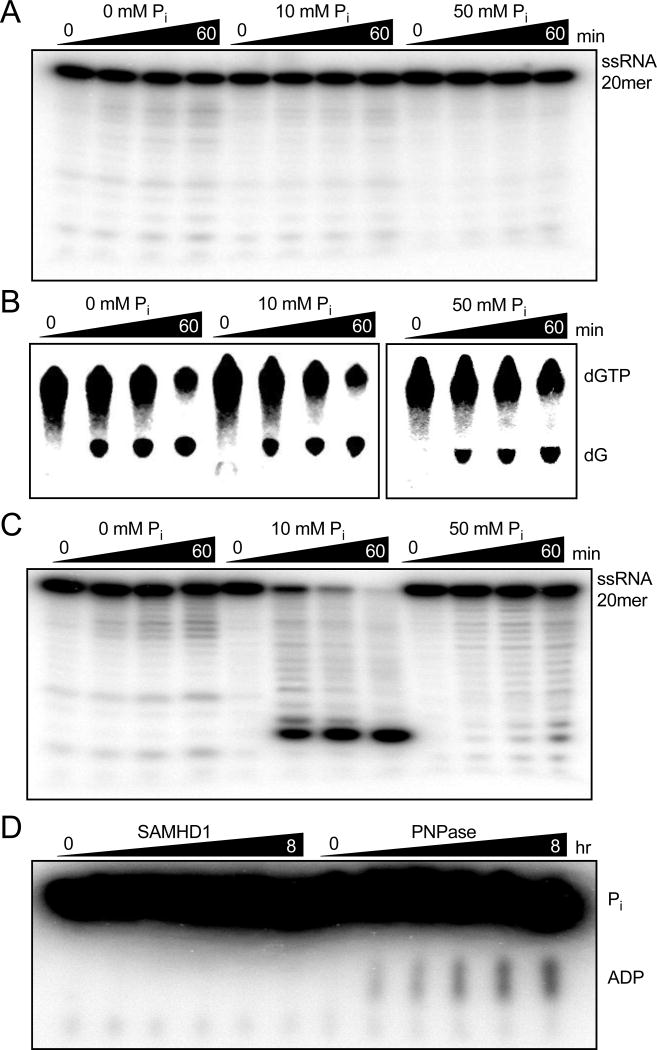
Figure 6. SAMHD1 does not posses hydrolytic or phosphorolytic RNase Activity.
(A) Reactions of 5’ 32P-labeled ssRNA 20mer (1 µM) and SAMHD1 (0.5 µM) were carried out in Tris/KCl/MgCl2 buffer supplemented with the indicated concentration of phosphate and degradation products were resolved by denaturing PAGE. (B) Reactions of 3H-dGTP (1 mM) and SAMHD1 (0.5 µM) carried out under the same conditions as above and the products were resolved by RP-TLC. (C) Reactions were prepared as in (A) but with 0.1 µM human polynucleotide phosphorylase as a positive control. Robust phosphorolytic activity is observed with 10 mM Pi, but is inhibited by high concentrations of Pi as previously reported54 (D) Reactions prepared with Tris/KCl/MgCl2 buffer, 32Pi (10 mM), poly(A) RNA (1 mg/mL), and SAMHD1 (5 µM) or the positive control enzyme human polynucleotide phosphorylase (1 µM) were carried out and the ADP product was resolved from Pi by PEI-Cellulose TLC.