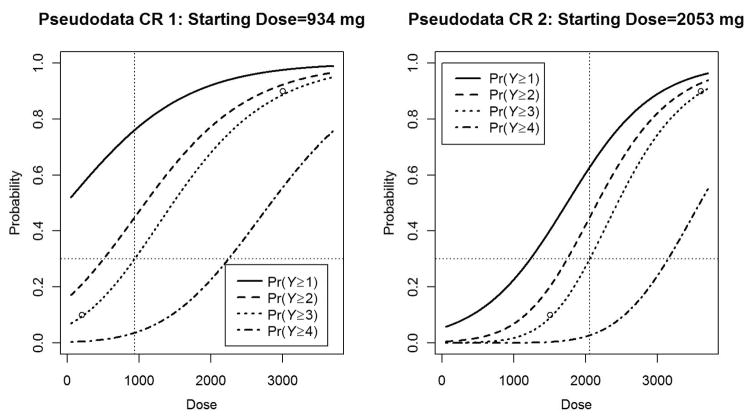
Figure 2.
From left to right: pseudodata CR 1 is utilized in scenarios A, C, and E and has a starting dose of 1060 mg for a 30% DLT rate. Pseudodata CR 2 is utilized in scenarios B, D, and F and has a starting dose equal to 2145 mg for a 30% DLT rate.
DLT: dose-limiting toxicity; CR: continuation ratio.