Abstract
Native, reassociated, and reconstituted core particles from chicken erythrocytes were compared by both biophysical and immunochemical methods. No significant difference between the three types of core particles could be demonstrated by electron microscopy, circular dichroism, or immunochemical analysis with antisera to histone H2B, H2A, and H3. Core particles were also reconstituted with calf thymus non-acetylated H3, H2A, and H2B with either mono-, di-, or tri-acetylated H4 isolated from cuttle -fish testes. The hyperacetylation of H4 did not significantly alter the biophysical characteristics of core particles but it induced several changes in their immunochemical reactivity. Binding to core particles of antibodies specific for H2A, H3, and for the IRGERA (synthetic C-terminal) peptide of H3 was considerably decreased when di- or tri-acetylated H4 was used for reconstitution, whereas binding of H2B antibodies remained the same. Our results suggest that the presence of hyperacetylated H4 within core particles leads to conformational changes that alter the antigenic determinants of several of the histones present at the surface of chromatin subunits. Since histone acetylation is correlated with the open structure of active chromatin, it may become possible to monitor the activity of chromatin by immunochemical methods.
Full text
PDF
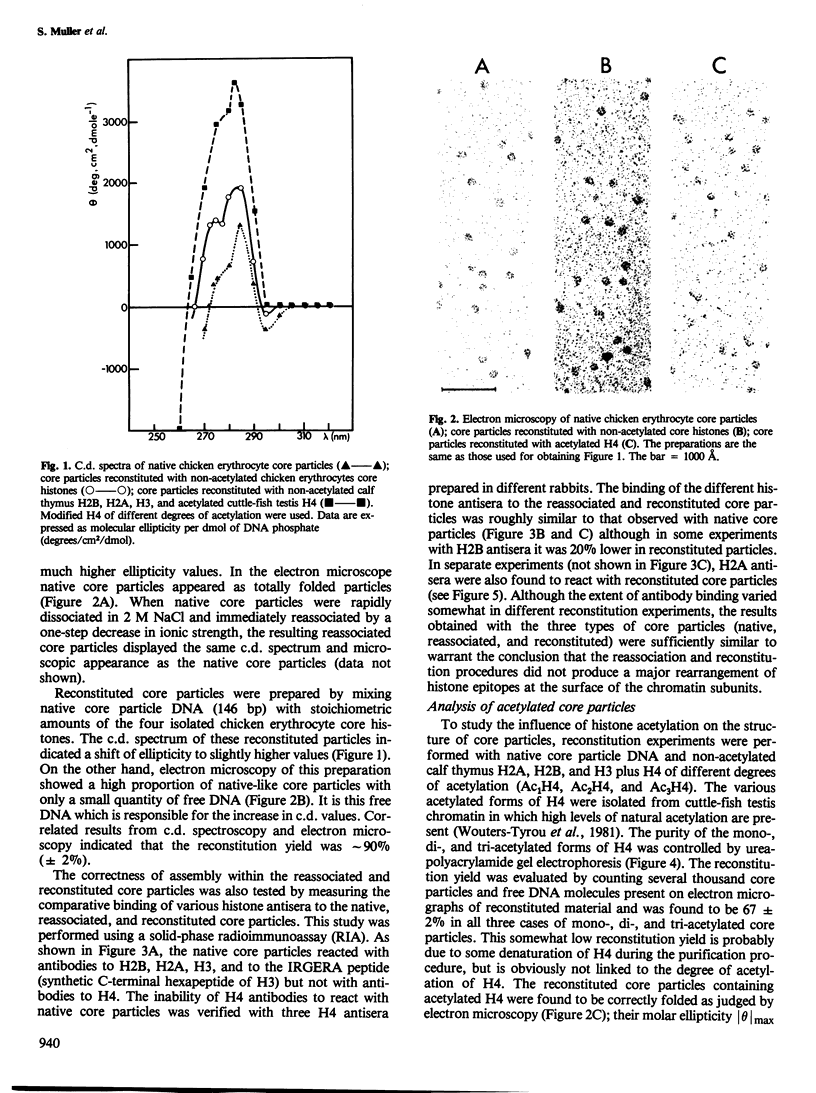
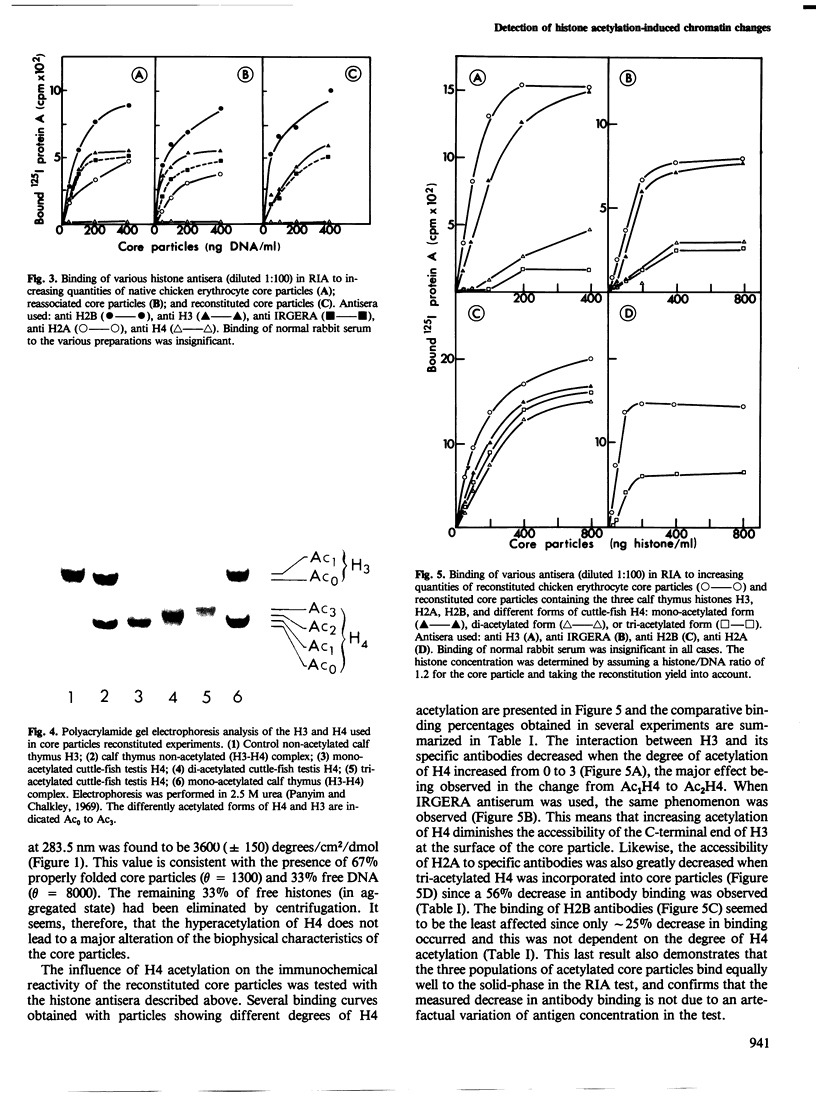
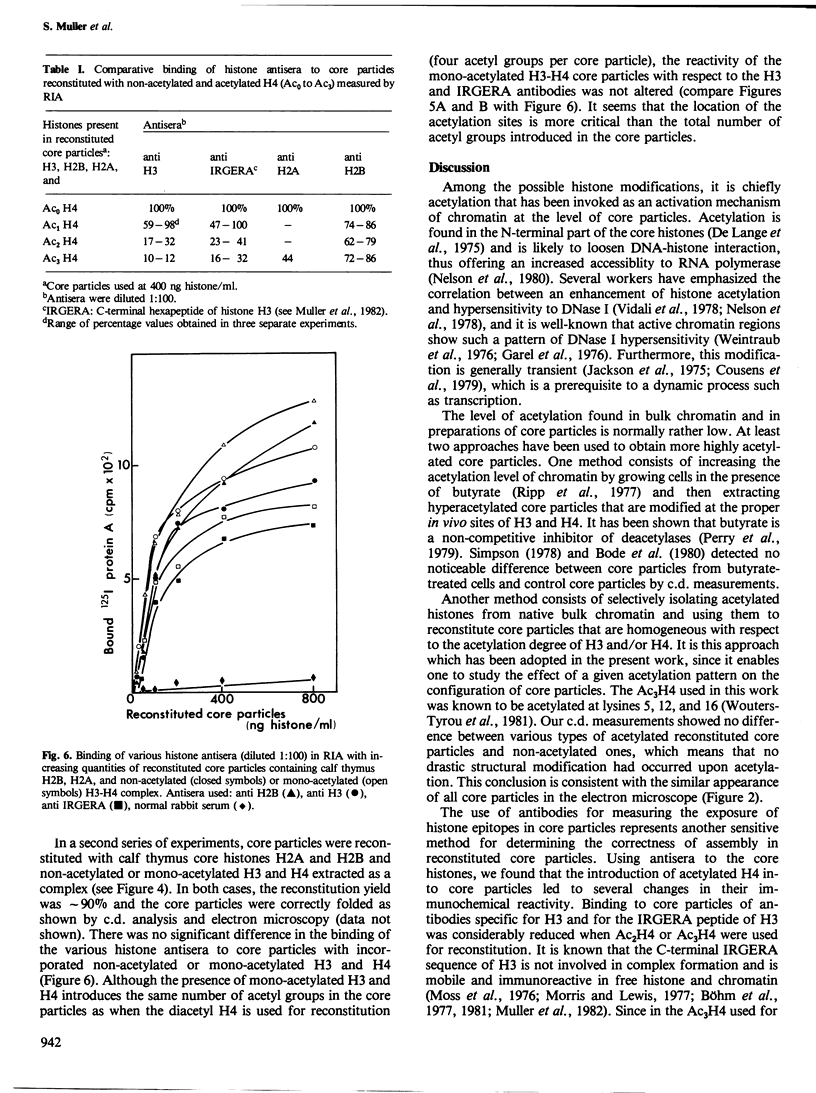


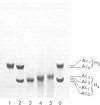
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D., Van Regenmortel M. H. Nucleosome structure studied with purified antibodies to histones H2B, H3 and H4. FEBS Lett. 1977 Dec 20;85(1):61–64. doi: 10.1016/0014-5793(78)81248-4. [DOI] [PubMed] [Google Scholar]
- Absolom D., van Regenmortel M. H. Purification by immunoadsorption and immunochemical properties of histone H3. FEBS Lett. 1977 Sep 15;81(2):419–422. doi: 10.1016/0014-5793(77)80568-1. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Smith J. A. A proposal for the nomenclature of antigenic sites in peptides and proteins. Immunochemistry. 1978 Aug;15(8):609–610. doi: 10.1016/0161-5890(78)90016-0. [DOI] [PubMed] [Google Scholar]
- Bode J., Henco K., Wingender E. Modulation of the nucleosome structure by histone acetylation. Eur J Biochem. 1980 Sep;110(1):143–152. doi: 10.1111/j.1432-1033.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Böhm L., Briand G., Sautière P., Crane-Robinson C. Proteolytic digestion studies of chromatin core-histone structure. Identification of the limit peptides of histones H3 and H4. Eur J Biochem. 1981 Sep;119(1):67–74. doi: 10.1111/j.1432-1033.1981.tb05577.x. [DOI] [PubMed] [Google Scholar]
- Böhm L., Hayashi H., Cary P. D., Moss T., Crane-Robinson C., Bradbury E. M. Sites of histone/histone interaction in the H3 - H4 complex. Eur J Biochem. 1977 Aug 1;77(3):487–493. doi: 10.1111/j.1432-1033.1977.tb11690.x. [DOI] [PubMed] [Google Scholar]
- Chahal S. S., Matthews H. R., Bradbury E. M. Acetylation of histone H4 and its role in chromatin structure and function. Nature. 1980 Sep 4;287(5777):76–79. doi: 10.1038/287076a0. [DOI] [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Shires A., Chalkley R., Granner D. K. Studies on highly metabolically active acetylation and phosphorylation of histones. J Biol Chem. 1975 Jul 10;250(13):4856–4863. [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Morris G., Lewis P. N. Conformational studies on histone H3 and its CNBr peptides. Eur J Biochem. 1977 Aug 1;77(3):471–477. doi: 10.1111/j.1432-1033.1977.tb11688.x. [DOI] [PubMed] [Google Scholar]
- Moss T., Cary P. D., Crane-Robinson C., Bradbury E. M. Physical studies on the H3/H4 histone tetramer. Biochemistry. 1976 Jun 1;15(11):2261–2267. doi: 10.1021/bi00656a003. [DOI] [PubMed] [Google Scholar]
- Muller S., Himmelspach K., Van Regenmortel M. H. Immunochemical localization of the C-terminal hexapeptide of histone H3 at the surface of chromatin subunits. EMBO J. 1982;1(4):421–425. doi: 10.1002/j.1460-2075.1982.tb01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura C. V., Mazen A., Neelin J. M., Briand G., Sautiere P., Champagne M. Distribution of antigenicity in chicken erythrocyte histone H5. Eur J Biochem. 1980 Jul;108(2):613–620. doi: 10.1111/j.1432-1033.1980.tb04756.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- Nelson D., Covault J., Chalkley R. Segregation of rapidly acetylated histones into a chromatin fraction released from intact nuclei by the action of micrococcal nuclease. Nucleic Acids Res. 1980 Apr 25;8(8):1745–1763. doi: 10.1093/nar/8.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlenbusch H. H. Structural complementarity in chromatin subunits. Naturwissenschaften. 1979 Apr;66(4):206–207. doi: 10.1007/BF00366027. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry M., Nelson D., Moore M., Chalkley R. Histone deacetylation in nuclei isolated from hepatoma tissue culture cells. Inhibition by sodium butyrate. Biochim Biophys Acta. 1979 Feb 27;561(2):517–525. doi: 10.1016/0005-2787(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Romac J., Bouley J. P., Van Regenmortel M. H. Enzyme-linked immunosorbent assay in the study of histone antigens and nucleosome structure. Anal Biochem. 1981 May 15;113(2):366–371. doi: 10.1016/0003-2697(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Sela M. Antigenicity: some molecular aspects. Science. 1969 Dec 12;166(3911):1365–1374. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Ward M. Rabbit antibodies to histone fractions as specific reagents for preparative and comparative studies. J Biol Chem. 1970 Mar 25;245(6):1261–1266. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Martin-Ponthieu A., Sautiere P., Biserte G. Acetylation of histone H4 in chicken erythrocyte and cuttle-fish testis chromatin. FEBS Lett. 1981 Jun 15;128(2):195–200. doi: 10.1016/0014-5793(81)80079-8. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Mazen A., Erard M., Pouyet J., Champagne M. Isolation and physical characterization of a stable core particle. Nucleic Acids Res. 1980 Feb 25;8(4):767–779. [PMC free article] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]