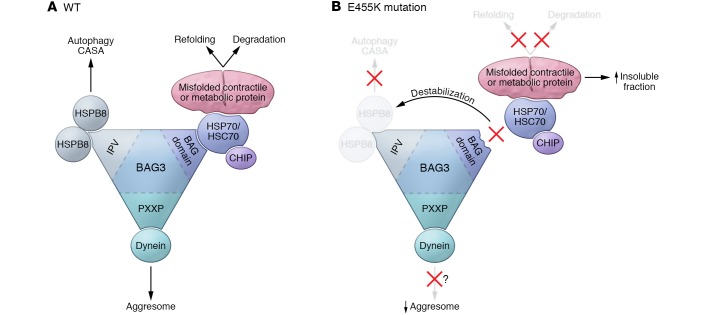
Figure 1. Putative functions of BAG3 in the heart and the underlying mechanisms of cardiomyopathy caused by loss of BAG3 function.
BAG3 plays a critical role in the PQC system in the heart. WT BAG3 forms multi-chaperone complexes with HSP70/HSC70 and sHSPs via its IPV motif and BAG3 domain. The BAG3-HSPB8-HSC70 complex binds to misfolded proteins and either promotes refolding or degradation of these misfolded proteins. Degradation is mediated primarily through general autophagy. The BAG3-HSPB8-HSC70 complex also maintains the structure and integrity of sarcomeres via CASA, a substrate-specific form of autophagy. In addition, BAG3 binds to dynein via the proline-rich motif (PXXP) and segregates misfolded proteins into aggresomes located in the perinuclear space through retrograde transport. The BAG3E455K mutation, which is located in the middle of the BAG domain, attenuates the interaction between BAG3 and HSP70/HSC70, leading to destabilization of sHSPs, including HSPB8. This disturbance of the chaperone complexes induces dysregulation of the refolding and degradation processes (autophagy and CASA), resulting in accumulation of BAG3 complex substrate proteins involved in metabolism and cardiac contraction in the detergent-insoluble fraction. Interestingly, aggresomes are not obvious in the BAG3 loss-of-function mouse model, possibly because of the defect in retrograde transport. Despite the absence of large protein aggregates, BAG3 loss-of-function mice develop DCM.