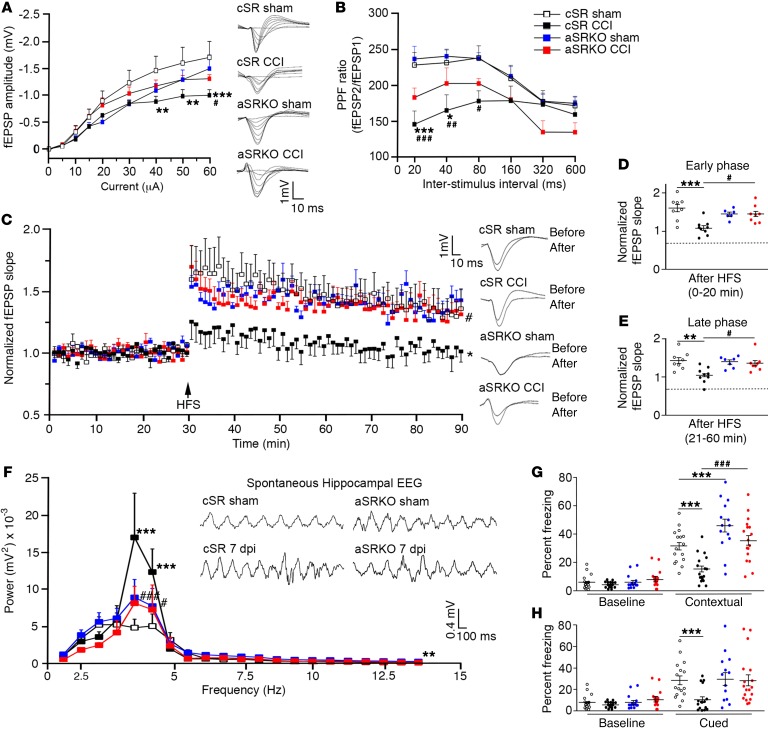
Figure 4. Synaptic dysfunction and learning deficits are not observed in aSRKO CCI-injured mice at 7 dpi.
(A) I/O curve showed reduced fEPSP amplitude at high intensities for cSR CCI-injured mice as compared with sham-treated and CCI-injured aSRKO mice. Individual traces shown. (B) PPF showed a reduced ratio for cSR CCI-injured mice as compared with sham-treated and CCI-injured aSRKO mice. (C) LTP was reduced in CCI-injured cSR control mice, but not sham-treated cSR, sham-treated aSRKO, or CCI-injured aSRKO mice. Individual traces shown. (D) Histograph of LTP early phase showed greatest reductions in CCI-injured cSR mice and reductions to a lesser extent in aSRKO mice. (E) Histograph of LTP late phase showed greatest reductions in CCI-injured cSR mice and reductions to a lesser extent in aSRKO mice. Early and late phase slopes of CCI-injured aSRKO mice were significantly greater than CCI-injured cSR mice at 7 dpi. (F) θ rhythm frequency was increased in CCI-injured cSR mice and was increased to a lesser extent in sham-treated and CCI-injured aSRKO mice at frequencies between 4 and 5 Hz as compared to sham-treated cSR mice. θ rhythm frequency of CCI-injured aSRKO mice were significantly less than CCI-injured cSR mice. θ rhythm traces were shown for amplitude versus time. Fear-conditioning tests showed significantly decreased freezing behavior in CCI-injured cSR mice for contextual (G) and cued (H) learning that was not observed in sham-treated or CCI-injured aSRKO mice. Data represent mean ± SEM. (A–F) n = 7–10/group. (G and H) n = 16–18/group. (A–C, F–H) Two-way RM ANOVA. (D and E) One-way ANOVA. *P < 0.05, **P < 0.01; *** P < 0.001, compared with cSR control mice. #P < 0.05; ##P < 0.01; ###P < 0.001, compared with cSR at 7 dpi.