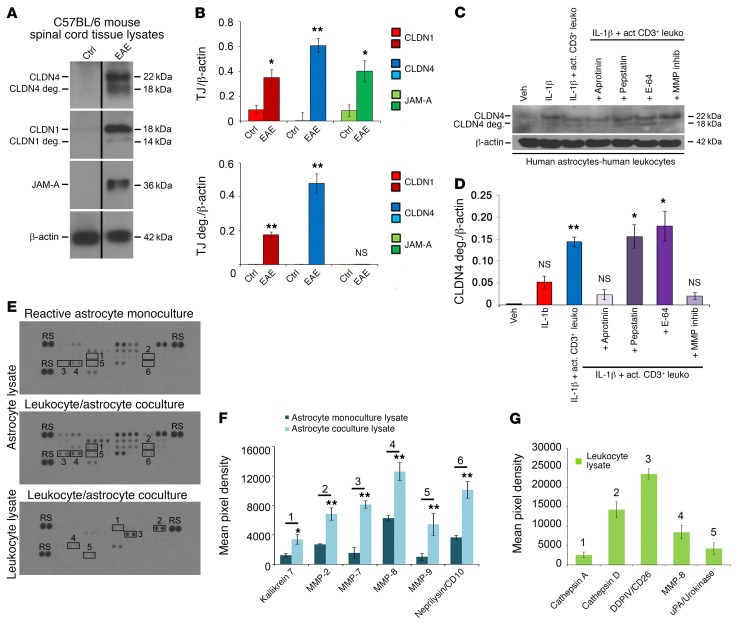
Figure 7. Astrocytic CLDN4 is degraded in EAE lesions and in coculture with activated CD3+ lymphocytes in vitro.
(A and B) Immunoblotting (A) and densitometric quantification (B) of spinal cord lysates from C57BL/6 mice with EAE (score 2–3, from 18–21 days) and age- and sex-matched controls demonstrate induction of CLDN1, CLND4, and JAM-A in EAE (A and B, upper panel), along with degradation products of CLDN1 and CLDN4 (14 kDa and 18 kDa), but not JAM-A (A and B, lower panel). (C and D) Immunoblotting and densitometry of cocultures of reactive human astrocytes (IL-1β–treated followed by washout) with activated CD3+ lymphocytes. Coculture leads to degradation of astrocytic CLDN4 by 24 hours, and CLDN4 degradation is blocked by specific protease inhibitors, including the serine protease inhibitor aprotinin, and by MMP inhibitor-2. In contrast, degradation is not blocked by the cysteine protease inhibitor E-64, or the aspartic protease inhibitor pepstatin. These studies collectively suggest combinations of kallikrein and urokinase (substrates of aprotinin) and MMP-1, -3, -7, and -9 (substrates of MMP inhibitor-2) as potentially responsible for CLDN1 and CLDN4 digestion (see also Supplemental Figure 5, A–C). (E–G) Human astrocytes were pretreated for 24 hours with 10 mg/ml IL-1β, then washed and cultured alone or with activated CD3+ cells for 72 hours. Supernatant and cell lysates of isolated astrocytes or leukocytes were then harvested and applied to protease arrays (3 biological replicates of each condition) (E and F). Astrocyte lysates from coculture with CD3+ cells showed upregulation of kallikrein 7, MMP-2, -7, -8, and -9, and CD10 compared with lysate from monoculture (n = 3 each group, 2-tailed t test, P < 0.05). (E and G) CD3+ cell lysates from coculture demonstrated strongest expression of cathepsins A and D, DDPIV, MMP-8, and uPA. See also Supplemental Figure 5, D and E. Data in A–G are representative of findings from 3 or more biological replicates. *P < 0.05, **P < 0.01.