Abstract
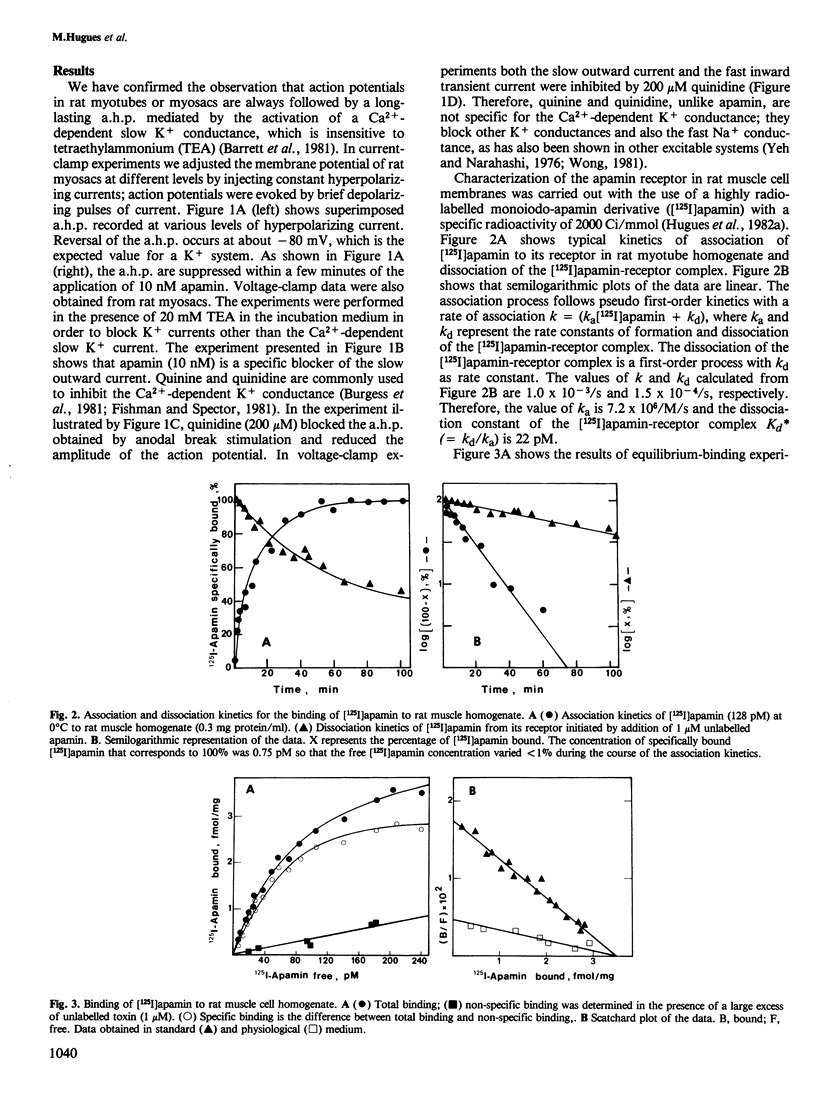
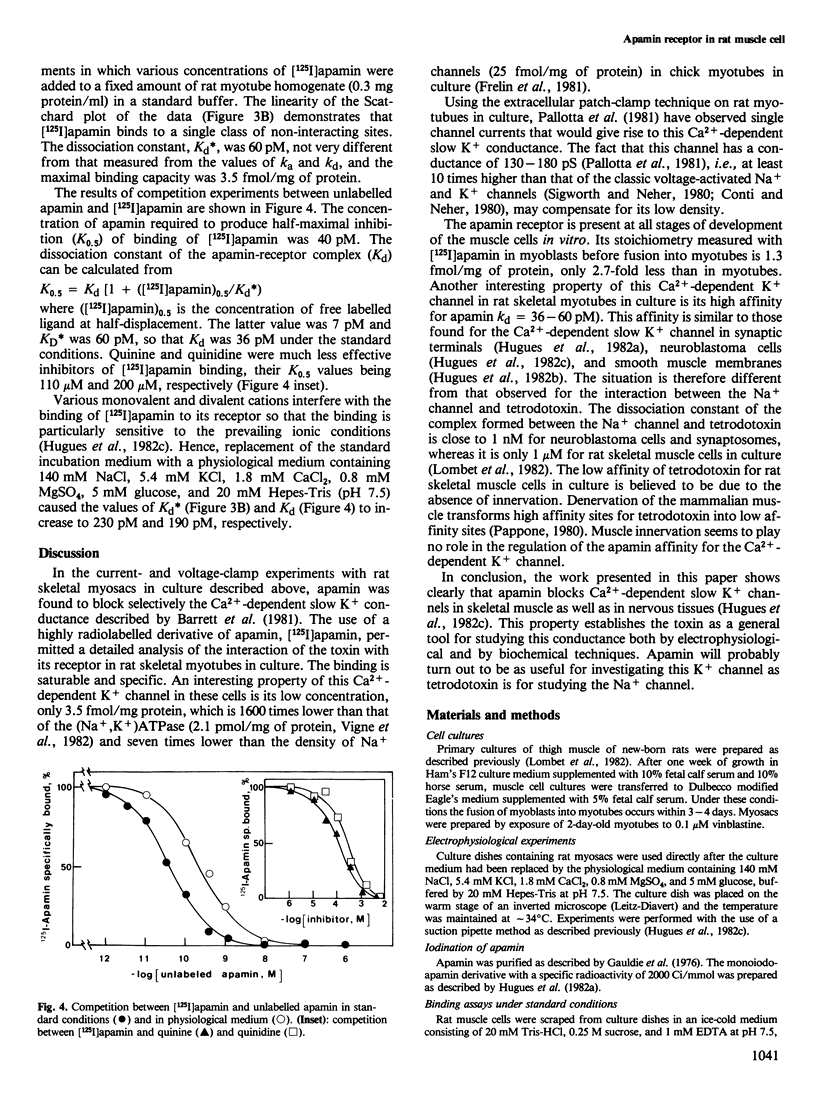
The interaction of apamin, a bee venom neurotoxin, with rat skeletal muscle cell membranes has been followed using both an electrophysiological and a biochemical approach. Voltage-clamp analyses have shown that apamin, at low concentrations, specifically blocks the Ca2+-dependent slow K+ conductance in rat myotubes and myosacs . A specific binding site for apamin in rat muscle cell membranes has been characterized with the use of a highly radiolabelled apamin derivative [( 125I]apamin). The dissociation constant for the apamin-receptor complex is 36-60 pM and the maximal binding capacity is 3.5 fmol/mg of protein. [125I]Apamin binding to rat muscle membranes is displaced by quinine and quinidine with K0.5 values of 110 microM and 200 microM, respectively.
Full text
PDF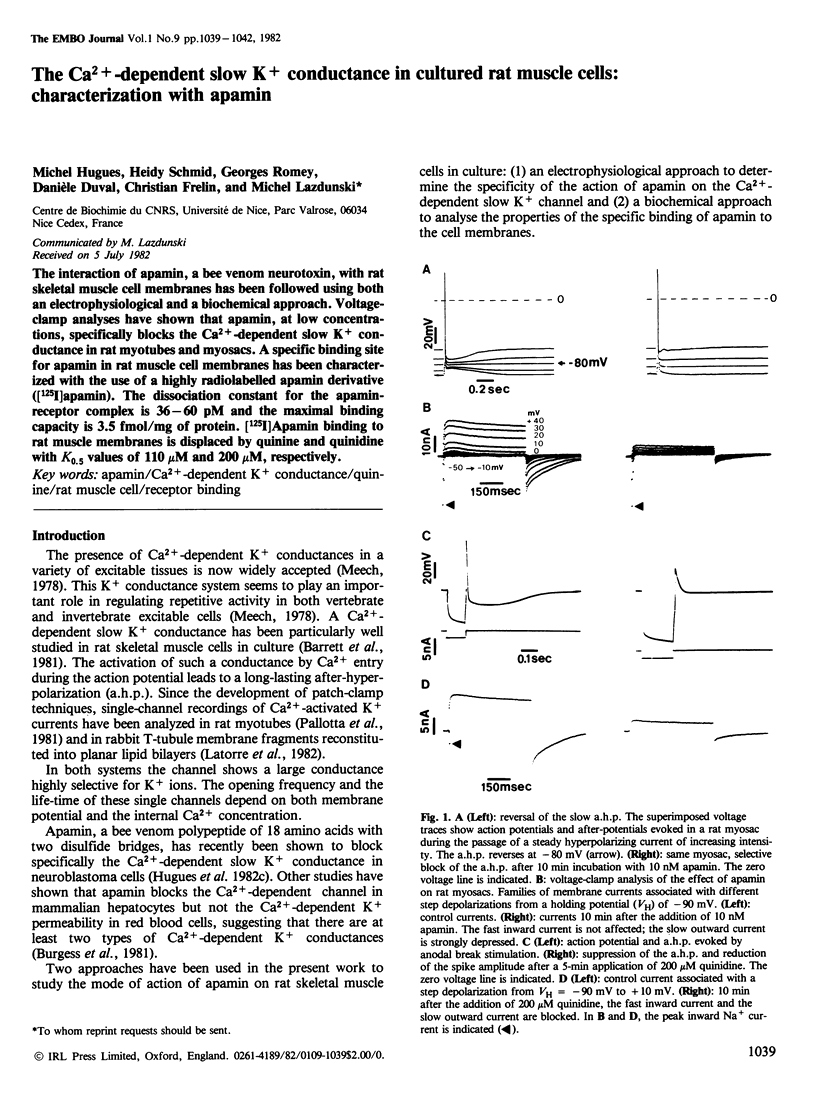

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Barrett E. F., Dribin L. B. Calcium-dependent slow potassium conductance in rat skeletal myotubes. Dev Biol. 1981 Mar;82(2):258–266. doi: 10.1016/0012-1606(81)90450-4. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Lombet A., Vigne P., Romey G., Lazdunski M. The appearance of voltage-sensitive Na+ channels during the in vitro differentiation of embryonic chick skeletal muscle cells. J Biol Chem. 1981 Dec 10;256(23):12355–12361. [PubMed] [Google Scholar]
- Gauldie J., Hanson J. M., Rumjanek F. D., Shipolini R. A., Vernon C. A. The peptide components of bee venom. Eur J Biochem. 1976 Jan 15;61(2):369–376. doi: 10.1111/j.1432-1033.1976.tb10030.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hugues M., Duval D., Kitabgi P., Lazdunski M., Vincent J. P. Preparation of a pure monoiodo derivative of the bee venom neurotoxin apamin and its binding properties to rat brain synaptosomes. J Biol Chem. 1982 Mar 25;257(6):2762–2769. [PubMed] [Google Scholar]
- Hugues M., Duval D., Schmid H., Kitabgi P., Lazdunski M., Vincent J. P. Specific binding and pharmacological interactions of apamin, the neurotoxin from bee venom, with guinea pig colon. Life Sci. 1982 Aug 2;31(5):437–443. doi: 10.1016/0024-3205(82)90328-9. [DOI] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Vergara C., Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):805–809. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombet A., Frelin C., Renaud J. F., Lazdunski M. Na+ channels with binding sites of high and low affinity for tetrodotoxin in different excitable and non-excitable cells. Eur J Biochem. 1982 May;124(1):199–203. doi: 10.1111/j.1432-1033.1982.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Lazdunski M. Ontogeny of the (Na+,K+)-ATPase during chick skeletal myogenesis. J Biol Chem. 1982 May 25;257(10):5380–5384. [PubMed] [Google Scholar]
- Wong B. S. Quinidine interactions with Myxicola giant axons. Mol Pharmacol. 1981 Jul;20(1):98–106. [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Mechanism of action of quinidine on squid axon membranes. J Pharmacol Exp Ther. 1976 Jan;196(1):62–70. [PubMed] [Google Scholar]


