Abstract
The effect of the adenosine A1 receptor agonist N6-cyclopentyladenosine (CPA) and antagonist 8-cyclopentyl-l,3-dipropylxanthine (CPX) on N-methyl-d-aspartate (NMDA)-evoked seizures was studied in C57BL/6 mice (20/group). Animals were injected i.p. either with CPA (0.5, 1, 2 mg/kg) or CPX (1, 2 mg/kg) 15 min prior to administration of NMDA (30, 60, 125 mg/kg). Administration of NMDA alone resulted in a complete locomotor arrest at 30 mg/kg, while clonic/tonic seizures and progressively increasing mortality were seen at higher doses. Prior administration of CPA resulted either in a delay of seizure onset and unchanged mortality (0.5 mg/kg CPA, 60 mg/kg NMDA) or in elimination of tonic episodes and a significant reduction in postictal mortality (1, 2 mg/kg CPA; 60, 125 mg/kg NMDA). Pretreatment with CPX at either 1 or 2 mg/kg eliminated locomotor depression in animals injected with NMDA at 30 mg/kg. At 60 mg/kg NMDA, the effect of CPX administration resulted in mortality equivalent to that seen with 125 mg/kg NMDA administered alone. The results indicate that A1 receptor agonists may protect against NMDA-evoked seizures and that the adenosine A1 receptor may be directly involved in these actions.
Keywords: Adenosine receptor agonist, Adenosine receptor antagonist, Seizure, NMDA (N-methyl-d-aspartate), Protection, (Mouse)
1. Introduction
Adenosine is one of the major neuromodulators in the central nervous system (CNS) (for a recent review, see Daval et al., 1991). The neuromodulatory effects of adenosine are promoted through stimulation of two major receptors subtypes, A1 and A2. Activation of the A1 site results in inhibition of adenylate cyclase, while activation of the A2 receptor results in its stimulation. Ion channels and phosphatidyl inositol breakdown can also be affected. Among the most prominent CNS actions of adenosine and its stable analogues are their ability to inhibit the release of excitatory amino acids and other neurotransmitters, and their ability to inhibit spontaneous firing of neurons (for a review, see Ribeiro, 1991).
The notion that adenosine may provide an ‘inhibitory tone’ in the mammalian CNS (Harms et al., 1978) received broad experimental support. It has been also suggested that it may act as an endogenous anticonvulsant (Dragunow et al., 1985), and several studies showed that A1 receptor agonists reduce chemically and electrically stimulated epileptic seizures (Barraco et al., 1986; Murray and Szot, 1986; Murray et al., 1985). Furthermore, it has been also demonstrated that levels of endogenous adenosine increase during peripheral and central trauma (Berne, 1963; Berne et al., 1974). Since adenosine and its analogues depress the release of excitatory amino acids, compounds active at the A1 site also act as neuroprotectants against excitotoxic damage caused by hyperactivation of NMDA receptors seen in cerebral ischemia and stroke (Dragunow and Faull, 1988). Such protection has been demonstrated by several authors (for a review, see Miller and Hsu, 1992). Recent evidence (Schubert et al., 1992) indicates that, due to its inhibition of Ca2+ influx, adenosine may play a critical role in determination of input frequencies necessary for activation of NMDA receptors. Consequently, protective effects of adenosine in stroke and cerebral ischemia may be related to the diminished release of excitatory amino acids and to events triggered by postsynaptic activation of A1 sites. Moreover, adenosine-mediated postsynaptic modulation of neuronal activity indicates a possibility that prior administration of A1 analogues may either reduce the intensity or completely prevent seizures induced by NMDA. The present study explores this hypothesis.
2. Materials and methods
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) weighing approximately 30 g each were used in the study. All drugs were purchased from Research Biochemicals (Natick, MA). N6-Cyclopentyladenosine (CPA) and 8-cyclopentyl-l,3-dipropylxanthine were dissolved in a 20 : 80 mixture of Alkamuls EL-620 (Rhone-Poulenc, Cranbury, NJ) and saline, while NMDA was dissolved in buffered saline. Drug solutions (0.15 ml) were injected i.p. (n = 20/group) using a 25 gauge needle at the following doses: NMDA 30, 60 and 125 mg/kg; CPA 0.5, 1 and 2 mg/kg; CPX 1 and 2 mg/kg. CPA and CPX were administered 15 min prior to NMDA. To exclude a possibility of motor disturbances caused by either CPA or CPX, mice were tested on a rotorod revolving at 5 rpm for 2 min immediately before injection of NMDA. Immediately after injection of NMDA, animals were placed in a transparent cage and the incidence and type of convulsions, of other abnormal behaviour patterns, and mortality were observed for the following 5 h. All animals whose locomotor behaviour was depressed during the observation period were also tested every 30 min on the rotorod set at 5 rpm.
In order to eliminate the possibility that CPA-evoked hypothermia might have an ameliorative effect of its own and could, therefore, contribute to the reduction of the incidence/intensity of seizures, behaviour of four separate drug-injected groups of animals (n = 10/group) was studied in a translucent chamber whose ambient temperature was maintained at 42°C by means of a water bath. Following 15 min acclimatization in the chamber, rectal temperature of each animal was measured using an electronic rectal probe (Harvard, S. Natick, MA). Scalp surface temperature was measured using an infrared veterinarian thermometer (Exergen, Newton, MA) held at a distance of 2 mm from the surface of the scalp (table 1). Immediately thereafter, animals were injected with either CPA (1 mg/kg), CPX (1 mg/kg), NMDA (60 mg/kg), or CPA (1 mg/kg) followed 15 min later by NMDA (60 mg/kg), and put again into the chamber for the following 2 h. Since NMDA-induced convulsions precluded safe insertion of the rectal probe in unanaesthetized mice, and since tactile stimulation could result in spuriously induced seizures, all subsequent temperature measurements (at 15, 30 and 120 min after injections) were made using the infrared device. Following 2 h in the chamber, the animals were placed in their home cages kept at the room temperature and their behaviour was observed for the subsequent 3 h.
TABLE 1.
The effect of 1 mg/kg CPA or 60 mg/kg NMDA on rectal and scalp temperature of animals maintained either at ambient temperature or at 42°C.
| t/amb | t/15 | t/30 | t/120 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Control | NMDA | CPA | Control | NMDA | CPA | Control | NMDA | CPA | Control | NMDA | CPA | |
| Rectal | 39.9±0.2 | n.m. | 37.3±0.1 a | 39.2±0.2 | n.m | 38.6±0.5 | 40.1±0.3 | n.m. | 39.3±0.3 | 40.6±0.8 | n.m | 40.0±0.6 |
| Scalp | 30.2±0.3 | 30.1±0.4 | 27.9±2.5 a,b | 30.0±0.4 | 29.3±0.7 | 31.1±0.6 | 32.1±0.7 | 32.8±1.5 | 33.2±0.4 | 32.3±0.5 | 33.0±0.9 | 32.6±1.4 |
Values are mean ± S.E.M. t/amb: ambient temperature (23.1°C), values measured 15 min after injection of either drug; t/15, t/30, t/120: controlled environment, values measured at 15, 30, 120 min post-injections.
Significance (ambient temperature groups): P < 0.05,
, compared to control;
, between treatment groups. No significant changes in the controlled environment groups. Bonferroni’s corrected Student’s t-test.
In each group, the severity of neurological impairment following drug treatment was graded on a 15 point scale (table 2). Statistical significance of differences in the incidence and delay of seizures, degree of neurological impairment, and time to death were evaluated using Bonferroni’s corrected Student’s t-test, while percent incidence of seizures and other forms of neurological impairment, and the end-point survival data were determined using Fisher’s exact test. P > 0.05 was considered significant.
TABLE 2.
Neurological impairment scale.
| No change | 0 |
| Depression | 1 |
| Scratching/biting | 2 |
| Hyperactivity | 3 |
| Clonic seizures | 4 |
| Clonic/tonic complexes | 5 |
3. Results
3.1. The effect of CPA and CPX
Injections of CPA alone produced complete immobility lasting from 1.5 h (0.5 mg/kg) to over 12 h (2 mg/kg). At lower doses (0.5 and 1 mg/kg) there was no motor coordination effect of the drug and all animals stayed indefinitely on the rotorod. At 2 mg/kg this number was reduced to 80%. Administration of CPA at 1 mg/kg resulted in the reduction of scalp temperature by 2.5 ± 0.3°C (P < 0.05) at 15, 30, and 120 min after the injection. On the other hand, CPX produced no motor coordination or temperature effect at 1 mg/kg, and no motor coordination effects at 2 mg/kg.
3.2. Administration of NMDA alone
Administration of NMDA at 30 mg/kg resulted in a complete arrest of spontaneous locomotion and sedation in 90% animals (table 3). However, although sharp sound did not produce any visible reaction, when touched, all locomotor-depressed animals responded by shifting to a new position. Rotorod testing did not reveal any motor deficits, and the scalp temperature remained unchanged. Normal behaviour returned between 2 and 3 h after administration of the drug. In 10% animals injected with 30 mg/kg NMDA, clonic seizures developed within 10 min after the injection (table 4).
TABLE 3.
Percent incidence of seizures and/or other neurological symptoms following administration of various doses of either NMDA alone or CPA or CPX followed by NMDA.
| Dose mg/kg |
Clon/ton | Clon | Hyperact | Depr | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| NMDA | CPA + NM | NMDA | CPA + NM | NMDA | CPA + NM | NMDA | CPA + NM | |
| 2A/125NM | 90 | 10 a | 10 | 40 a | 0 | 0 | 0 | 50 |
| 0.5A/60NM | 40 | 10 a | 50 | 0 a | 10 | 70 a | 0 | 20 |
| IA/60NM | 40 | 10 a | 30 | 0 | 30 | 50 | 0 | 20 |
| 1A/30NM | 10 | 0 | 0 | 0 | 0 | 0 | 90 | 100 |
|
| ||||||||
| NMDA | CPX + NM | NMDA | CPX + NM | NMDA | CPX + NM | NMDA | CPX + NM | |
|
| ||||||||
| 1X/60NM | 40 | 60 | 30 | 10 a | 30 | 30 | 0 | 0 |
| 2X/60NM | 40 | 70 a | 30 | 10 a | 30 | 20 a | 0 | 0 |
| 1X/30NM | 0 | 0 | 0 | 10 a | 0 | 70 a | 100 | 20 a |
| 2X/30NM | 0 | 0 a | 0 | 10 a | 0 | 80 a | 100 | 10 a |
Abbreviations: clon/ton, clonic/tonic complex; clon, clonic seizures; hyperact, hyperactivity; depr, locomotor depression; NM, NMDA; A, CPA; X, CPX.
P < 0.05, Fisher’s exact text.
TABLE 4.
The effect of different doses of either CPA or CPX on the average latency of neurological symptoms, degree of neurological impairment, and mortality caused by i.p. administration of NMDA.
| Dose mg/kg |
Latency (s) | P | Impairment | P | T/death (h) | P |
|---|---|---|---|---|---|---|
| 30.0 NMDA | 542±97 | 1.9±0.8 | n.s. | 4.7 ±0.3 | ||
| 1.0 CPA ±30.0 NMDA | < 900 | < 0.05 | 1.0 ± 0.0 | n.s. | > 5 | n.s |
| 1.0 CPX ± 30.0 NMDA | 538 ±44 | n.s | 1.7 ± 0.1 | < 0.05 | > 5 | n.s. |
| 2.0 CPX ± 30.0 NMDA | 406 ± 66 | < 0.05 | 3.0 ± 0.8 | < 0.05 | > 5 | n.s. |
| 60.0 NMDA | 414±58 | 6.2±0.8 | 2.7 ±0.8 | |||
| 0.5 CPA ±60.0 NMDA | 467 ±51 | n.s. | 5.3 ± 1.1 | n.s. | 2.5 ± 0.6 | n.s. |
| 1.0 CPA± 60.0 NMDA | > 900 | < 0.05 | 2.1 ±0.6 | < 0.05 | 4.7 ±0.6 | < 0.05 |
| 1.0 CPX±60.0 NMDA | 249±54 | < 0.05 | 10.3±0.8 | < 0.05 | 2.0 ±0.7 | n.s |
| 2.0 CPX±60.0 NMDA | 138±28 | < 0.05 | 11.3±0.6 | < 0.05 | 0.8 ±0.4 | < 0.05 |
| 125.0 NMDA | 71 ± 5 | 11.5 ± 0.4 | < 0.05 | 0.68 ± 0.5 | ||
| 2.0 CPA ± 125.0 NMDA | 541 ± 47 | < 0.05 | 3.1 ±0.7 | < 0.05 | 3.65 ±0.7 | < 0.05 |
Values are mean ± S.E.M.
Latency: delay (s) to the first observable behavioral (seizure) symptoms. T/death: time to death (h).
P: Bonferroni’s corrected Student’s t-test, n = 20/group.
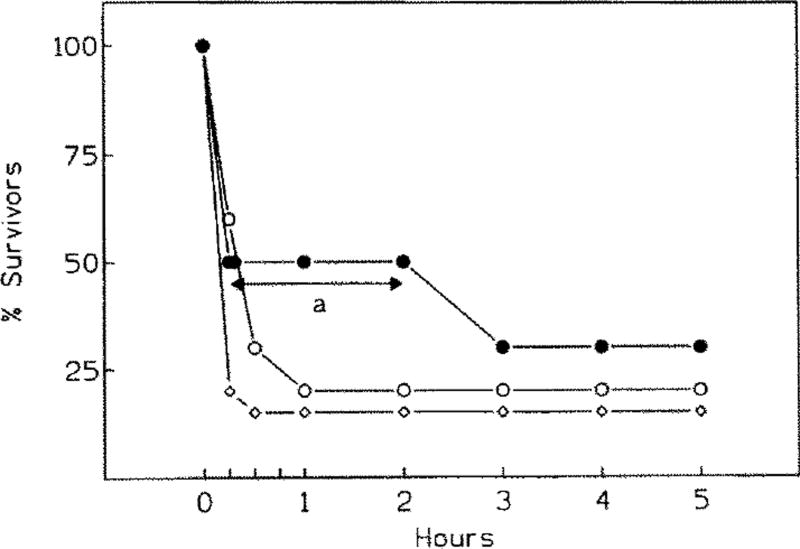
When NMDA was administered at 60 mg/kg, a brief period of hyperactivity ensued within 5 min after the injection in 30% animals (tables 3 and 4). In 30% mice, hyperactivity (running, jumping) transformed into clonic seizures, and in the remaining 40% animals into a clonic/tonic complex. In the latter case, death followed within 4–15 min thereafter (fig. 1).
Fig. 1.
Survival following administration of either NMDA alone (60 mg/kg, black circles), or of CPA (open triangles, 0.5 mg/kg; open squares, 1 mg/kg) given 15 min prior to 60 mg/kg NMDA. Note significant delay in the onset of seizures/mortality (a P > 0.05, Bonferroni’s corrected Student’s t-test) when CPA is given at 0.5 mg/kg.
Administration of NMDA at 125 mg/kg resulted in a very swift development of intense clonic/tonic seizures (table 4) followed by a rapid death of 90% animals (fig. 2).
Fig. 2.
Survival following administration of either NMDA alone (125 mg/kg, black circles), or of CPA (2 mg/kg, open squares) administered 15 min prior to NMDA.
3.3. Administration of CPA and NMDA
When 1.0 mg/kg CPA was given prior to 30 mg/kg NMDA, complete locomotor depression lasting 1.5–2 h developed in all animals within 10 min after injection of NMDA. Motor coordination was unaffected. No other doses of CPA were studied in combination with NMDA at 30 mg/kg.
Although treatment with 0.5 mg/kg CPA prior to NMDA at 60 mg/kg caused a significant (P < 0.05) reduction in the intensity of seizures (table 3), hyperactive behaviour was nonetheless present in 70% mice. Administration of CPA had no effect on either the degree of the overall neurological impairment or survival in this group (table 4 and fig. 1).
Significant reduction of the latency and incidence of clonic and tonic seizures was present in the group injected with 1 mg/kg CPA followed by 60 mg/kg NMDA (tables 3 and 4). Moreover, concomitant with the reduction of the overall neurological impairment (P < 0.05), survival at 5 h post-NMDA increased to 80% (P < 0.05, fig. 1). CPA given at 2 mg/kg prior to 125 mg/kg NMDA had equally significant impact upon all studied parameters (tables 3 and 4, fig. 2).
3.4. Temperature dependence of NMDA and CPA effects
Under conditions of controlled ambient temperature, there were no significant body temperature differences between animals injected with NMDA alone (60 mg/kg) or CPA (1 mg/kg) and NMDA (60 mg/kg). Nonetheless, seizure latency, the degree of neurological impairment, and the average time to death were all adversely affected in animals injected with NMDA alone (P < 0.05), while in the CPA/NMDA group only seizure latency was significantly reduced (table 5).
TABLE 5.
Comparison of the effect of 1 mg/kg CPA administered 15 min prior to 60 mg/kg NMDA on the latency and average neurological impairment, and mortality in animals maintained at the ambient temperature (uppermost row) and animals maintained in a constant temperature (42.0°C) environment (c).
| Dose mg/kg |
NMDA | CPA + NMDA | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Temp. (scalp) |
Latency (s) | Impairment | T/death (h) | Temp. (scalp) |
Latency (s) | Impairment | T/death (h) | ||
| 60 | 30.1±0.4 | 414±58 | 6.2±0.8 | 2.7±0.8 | 27.9±2.5 | > 900 | 2.1±0.6 | 4.7±0.6 | |
| 60 | c | 29.8±0.4 | 327±56 | 8.9±1.3 | 1.8±0.8 | 30.3±0.4 | > 900 | 2.0±0.8 | 4.5±0.4 |
| c | 33.4±0.6 | 33.0±1.1 | |||||||
| P | n.s. (a,b) | n.s. (a,b) | (n.s. a),b | (n.s. a),b | (n.s. a), b | (n.s. a),b | (n.s. a),b | ||
Values are mean ± S.E.M.
Significance: P < 0.05;
, within the treatment group;
, between treatment groups;
: animals maintained at 42°C (upper row – immediately before injections, lower row – 30 min after injections).
3.5. Administration of CPX and NMDA
Injection of 1 mg/kg CPX followed by 30 mg/kg NMDA transformed complete locomotor depression induced by 30 mg/kg NMDA into either hyperactivity (70% animals) or clonic seizures (10%) (fig. 3). In 20% mice, depression similar to that seen with NMDA alone was present (tables 3 and 4). Similar effects were produced by CPX at 2 mg/kg followed by 30 mg/kg NMDA.
Fig. 3.
Survival following administration of either CPX (1 mg/kg, open circles; 2 mg/kg, open diamonds) administered 15 rain prior to NMDA at 60 mg/kg, or of NMDA alone (black circles). Note that with NMDA alone, there is a transient of significantly lower mortality (a P > 0.05, Bonferroni’s corrected Student’s t-test).
CPX at either 1 or 2 mg/kg prior to 60 mg/kg NMDA led to a significant decrease in seizure latency and increased neurological impairment (tables 3 and 4). Moreover, administration of CPX at 2 mg/kg intensified the incidence of clonic/tonic complexes without, however, affecting the incidence of clonic seizures.
4. Discussion
Anticonvulsant actions of adenosine and its analogues have been described by several authors (reviewed by Dragunow, 1991). However, details of the mechanisms underlying these actions still remain unclear. Since excitatory amino acids are intimately involved in generation and propagation of epileptic activity (Dragunow, 1991), the inhibitory effects of exogenously applied adenosine (Dolphin and Archer, 1983; Coradetti et al., 1984) and of selective adenosine A1 receptor agonists (Fastbom and Fredholm, 1985) on the release of excitatory amino acids may play the major role in prevention of seizures.
Hoehn and White (1990) demonstrated that excessive concentration of extracellular glutamate can lead to a rapid, Ca2+-independent release of adenosine from rat cortical slices, and that this release is mediated through both NMDA and non-NMDA receptors. Although the lack of locomotor stimulation does not preclude the presence of electrographic seizure activity, the release of endogenous adenosine could provide an explanation for a surprisingly powerful depressant/sedative effect of NMDA administered at 30 mg/kg. Thus, a low dose of NMDA may have induced subthreshold receptor stimulation sufficient to trigger the release of endogenous adenosine. Although such hypothesis requires additional experiments, it is interesting that both the clinical picture (e.g., unaltered sensory responses, presence of startling and righting reflexes), and the time course of locomotor depression caused by CPA or other selective adenosine A1 receptor agonists observed in this and other studies (Von Lubitz and Marangos, 1990) were very similar to those seen with NMDA injected at 30 mg/kg.
The A1 selective agonist CPA had a powerful, temperature independent, inhibitory impact on seizure generation as demonstrated by a significant delay in the onset of convulsions and of seizure-related mortality. The A1 receptor appears to be involved in these actions since administration of CPX prior to intermediate doses of NMDA (60 mg/kg) had an opposite effect to that seen when CPA was given, and amplified both the intensity of convulsions and subsequent mortality to the degree observed when 125 mg/kg NMDA was injected alone. Furthermore, when CPX was given prior to 30 mg/kg NMDA, the NMDA-induced locomotor depression was transformed into intense hyperactivity or into bona fide clonic episodes.
Despite evidence that stimulation of A1 receptors may delay and significantly protect against NMDA-induced seizures, the exact mechanism of these effects remains to be clarified. It is unlikely that inhibition of amino acid release plays a major role since, with the doses of NMDA used in this study, the contribution of endogenously released excitatory amino acids would be, at best, minimal. Postsynaptic effects of the injected NMDA appear, therefore, to be the most prominent.
Adenosine has been shown to inhibit calcium fluxes (Schubert et al., 1986; Schubert, 1988) and open 4-aminopyridine-sensitive K+ channels (Schubert and Lee, 1986). Both of these actions would result in membrane hyperpolarization and an increase in threshold for the NMDA receptor activation. However, a systemic contribution of CPA to postepileptic survival cannot be excluded. Increased release of endogenous adenosine has been demonstrated during epileptic seizures (Schrader et al., 1980). Since adenosine and its analogues can improve cerebral blood flow (Van Wylen et al., 1991), the presence of CPA may have improved neuronal blood supply resulting in a better match between neuronal energy demand and supply during the critical, postictal period.
Whether the protection against NMDA-induced convulsions demonstrated in this study is species-specific or even strain-specific is unknown. In several previous studies (Ferkany et el., 1988; Leander et al., 1988; Skolnick et al., 1988) where different strains of mice were used, much higher doses of NMDA had to be used in order to produce lethality equivalent to that demonstrated by us in male C57BL/6 mice. Furthermore, we are unaware of any reports demonstrating that low doses of NMDA administered to other species or strains of mice result in a very pronounced locomotor depression, rather than the expected stimulation. The present observations indicate that adenosinergic agents may have potential applicability as anticonvulsants but additional studies of both mechanisms of action and of therapeutic outcome are needed.
References
- Barraco RA, Swanson TH, Phillis JW, Berman RF. Anticonvulsant effects of adenosine analogs on amygdaloid-kindied seizures in rats. Neurosci. Lett. 1986;46:317. doi: 10.1016/0304-3940(84)90118-6. [DOI] [PubMed] [Google Scholar]
- Berne RM. Cardiac nucleosides in hypoxia: possible role in regulation of coronary flow. J. Physiol. (London) 1963;204:317. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Berne RM, Rubio R, Cornish RR. Release of adenosine from ischemic brain. Circ. Res. 1974;32:262. [Google Scholar]
- Coradetti R, Lo Conte G, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur. J. Pharmacol. 1984;104:19. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- Daval J-L, Nehlig A, Nicolas F. Physiological and pharmacologial properties of adenosine: therapeutic implications. Life Sci. 1991;49:1435. doi: 10.1016/0024-3205(91)90043-b. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Archer ER. An adenosine agonist inhibits and a cyclic AMP analogue enhances the release of glutamate but not GABA from slices of rat dentate gyrus. Neurosci. Len. 1983;43:49. doi: 10.1016/0304-3940(83)90127-1. [DOI] [PubMed] [Google Scholar]
- Dragunow M. Adenosine and epileptic seizures. In: Phillis JW, editor. Adenosine and Adenine Nucleotides as Regulators of Cellular Function. CRC Press; Boca Raton: 1991. p. 367. [Google Scholar]
- Dragunow M, Faull RLM. Neuroprotective effects of adenosine. Trends Pharmacol. Sci. 1988;9:193. doi: 10.1016/0165-6147(88)90079-x. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Goddard GV, Laverty R. Is adenosine an endogenous anticonvulsant? Epilepsia. 1985;26:480. doi: 10.1111/j.1528-1157.1985.tb05684.x. [DOI] [PubMed] [Google Scholar]
- Fastbom J, Fredholm BB. Inhibition of [3H]glutamate release from rat hippocampal slices by l-phenylisopropyladenosine. Acta Physiol. Scand. 1985;125:121. doi: 10.1111/j.1748-1716.1985.tb07698.x. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Borosky SA, Clissold DB, Pontecorvo MJ. Dextromethorphan inhibits NMDA induced convulsions. Eur. J. Pharmacol. 1988;151:151. doi: 10.1016/0014-2999(88)90707-8. [DOI] [PubMed] [Google Scholar]
- Harms HH, Wardeh G, Mulder AH. Adenosine modulates depolarization induced release of [3H] noradrenaline from slices of rat brain neocortex. Eur. J. Pharmacol. 1978;49:305. doi: 10.1016/0014-2999(78)90107-3. [DOI] [PubMed] [Google Scholar]
- Hoehn K, White TD. N-Methyl-d-aspartate, kainate and quisqualate release endogenous adenosine from rat cortical slices. Neuroscience. 1990;2:441. doi: 10.1016/0306-4522(90)90280-h. [DOI] [PubMed] [Google Scholar]
- Leander JD, Lawson RR, Ornstein PL, Zimmerman DM. N-Methyl-d-aspartic acid induced lethality in mice: selective antagonism by phencyclidine-like drugs. Brain Res. 1988;448:115. doi: 10.1016/0006-8993(88)91107-9. [DOI] [PubMed] [Google Scholar]
- Miller LP, Hsu Ch. Therapeutic potential for adenosine receptor activation in ischemic brain injury. J. Neurotrauma. 1992;(Suppl. 2):$563. [PubMed] [Google Scholar]
- Murray TF, Szot P. A1 adenosine receptor mediated modulation of seizure susceptibility. In: Nistico G, editor. Neurotransmitters, Seizures, and Epilepsy. Raven Press; New York: 1986. p. 341. [Google Scholar]
- Murray TF, Sylvester D, Schultz CS, Szot P. Purinergic modulation of seizure threshold for pentylentetrazol in the rat. Neuropharmacology. 1985;24:761. doi: 10.1016/0028-3908(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA. Purinergic regulation of transmitter release. In: Phillis JW, editor. Adenosine and Adenine Nucleotides as Regulators of Cellular Function. CRC Press; Boca Raton: 1991. p. 155. [Google Scholar]
- Schrader J, Wahl M, Kuschinsky W, Kreutzberg GW. Increase of adenosine content in cerebral cortex of the cat during bicuculine induced seizures. Pflüg. Arch. Gesamte Physiol. Menschen Tiere. 1980;387:245. doi: 10.1007/BF00580977. [DOI] [PubMed] [Google Scholar]
- Schubert P. Modulation of synaptically evoked neuronal calcium fluxes by adenosine. In: Avoli M, Render TA, Dykes RW, Gloor P, editors. Neurotransmitters and Cortical Function: from Molecules to Mind. Plenum Press; New York: 1988. p. 471. [Google Scholar]
- Schubert P, Lee KS. Non-synaptic modulation of repetitive firing by adenosine is antagonized by 4-aminopyridine in rat hippocampal slice. Neurosci. Lett. 1986;67:334. doi: 10.1016/0304-3940(86)90332-0. [DOI] [PubMed] [Google Scholar]
- Schubert P, Heinemann U, Kolb R. Differential effects of adenosine on pre- and postsynaptic calcium fluxes. Brain Res. 1986;376:382. doi: 10.1016/0006-8993(86)90204-0. [DOI] [PubMed] [Google Scholar]
- Schubert P, Ferroni S, Mager R. Adenosine determines the critical input frequency for NMDA receptor-mediated neuronal Ca2+ influx. Int. J. Purine Pyrimidine Res. 1992;1:31. [Google Scholar]
- Skolnick P, Marvizon J, Jackson B, Monn J, Rice K, Lewin A. Blockade of N-methyl-d-aspartate induced convulsions by 1-aminocyclopropanecarboxylates. Life Sci. 1989;45:1647. doi: 10.1016/0024-3205(89)90274-9. [DOI] [PubMed] [Google Scholar]
- Van Wylen DGL, Sciotti VM, Winn HR. Adenosine and the regulation of cerebral blood flow. In: Phillis JW, editor. Adenosine and Adenine Nucleotides as Regulators of Cellular Function. CRC Press; Boca Raton: 1991. p. 191. [Google Scholar]
- Von Lubitz DKJE, Marangos PJ. Cerebral ischemia in gerbils: postischemic administration of cyclohexyl adenosine and 8-sulphophenyl-theophylline. J. Mol. Neurosci. 1990;2:53. doi: 10.1007/BF02896926. [DOI] [PubMed] [Google Scholar]